Cancer cells are often a mess of mutations. About 20% to 25% of cancers involve mutations in a complex of molecules called SWI/SNF. Yet drugs designed to block SWI/SNF activity haven’t always worked as expected. Researchers at Harvard Medical School have now figured out why.
As reported in an article published Nov. 2 in Cell, the team found that when drugs block SWI/SNF, a second molecule steps up to compensate.
Blocking this second molecule alongside SWI/SNF suppressed cancer cell growth in lab dishes, suggesting that a two-drug approach could make treatments more effective in people.
“I am excited about this work because it shows an alternative path forward for treating cancers in which the SWI/SNF complex is mutated,” said senior author Karen Adelman, the Edward S. Harkness Professor of Biological Chemistry and Molecular Pharmacology in the Blavatnik Institute at HMS, whose lab conducted the work.
“What’s interesting and meaningful about this study is it shows that a one-two punch, a double-agent therapy, could be really useful for keeping these cancer cells at bay,” she said.
Suppressing abnormal gene activity
The team made its discovery by answering questions about exactly how SWI/SNF works in both healthy and cancerous cells.
“If you understand exactly what the SWI/SNF complex is doing in normal cells you can find other vulnerabilities that show you how to do a better job of killing the cancer cells,” said Adelman. “Understanding basic mechanisms really helps you develop better therapeutic strategies.”
In human cells, DNA gets tightly packed into a form called chromatin that looks like a microscopic string of pearls. Various molecules called chromatin remodelers unspool chromatin in different spots, exposing certain genes so they can be used, and then repack the chromatin to tuck the genes away again.
SWI/SNF is one such chromatin remodeler. Researchers knew that it moves around the pearls, or nucleosomes, on the chromatin string—sliding them forward and back and sometimes plucking them off altogether—to control access to genes.
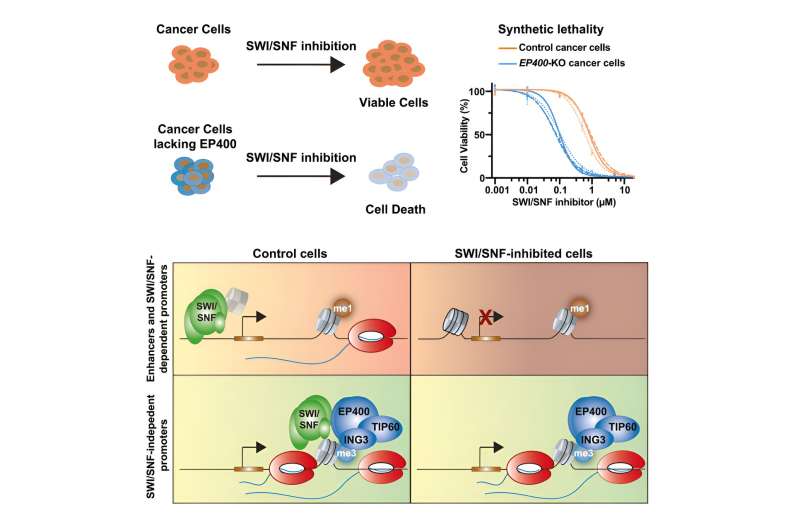
SWI/SNF treatments are supposed to suppress cancer-driving gene activity by stopping the mutated complex from providing inappropriate access to genes.
Study co-first authors Benjamin Martin, research fellow in biological chemistry and molecular pharmacology in the Adelman lab, and Eileen Ablondi, then a Ph.D. student in Harvard’s Biological and Biomedical Sciences Program, found that it’s not so simple. Blocking SWI/SNF suppressed the activity of only a subset of genes.
“When we inhibited SWI/SNF, at first all the genes turned off. Then most turned back on,” Adelman said.
The team probed further and discovered that another molecule, EP400, kicks in to restore gene access.
Only by blocking both SWI/SNF and EP400 could the researchers successfully suppress abnormal gene activity.
The type of cancer didn’t seem to matter, the team found. The two-punch treatment worked in eight patient cell lines representing four kinds of cancer: acute myeloid leukemia, diffuse intrinsic pontine glioma (DIPG), prostate cancer, and non-small cell lung cancer. Some of these lines had mutations in SWI/SNF and some did not.
“Part of the strength of the study is that the one-two punch can be used even in cancer cells with normal SWI/SNF,” said Adelman.
From basic science to new medicines
Adelman and colleagues are interested in exploring a SWI/SNF-EP400 combination therapy or seeing whether targeting one molecule is enough in cancers where the other molecule is already mutated.
“In parallel, we want to demonstrate that the combination works in principle by using a SWI/SNF blocker in tissue samples from patients who already have faulty EP400,” Adelman said. “The long-term goal is to have a safe and effective therapy for cancer patients with SWI/SNF mutations.”
The team has already made headway in predicting which genes in which cell types will be most susceptible to such a treatment.
“We developed a simple genomic assay, a computational approach, to rank the cell types and disease states in which the SWI/SNF blocker might be most useful,” said Adelman.
The work demonstrates the benefits of academic scientists’ partnering with industry, the authors pointed out. Adelman’s team achieved its insights because the company Novartis provided its SWI/SNF drug for the experiments. Now, those insights could help companies develop more effective cancer treatments.
Exploring basic biological questions is all the more satisfying when the answers could translate to new medicines, Adelman said.
“This is exactly what I like to do: start with a geeky question, then dig deep and come up with something that has broader implications,” she said.
More information:
Benjamin J.E. Martin et al, Global identification of SWI/SNF targets reveals compensation by EP400, Cell (2023). DOI: 10.1016/j.cell.2023.10.006
Journal information:
Cell
Source: Read Full Article