A study led by Daniel Jane-Wit, MD, Ph.D., a Yale School of Medicine associate professor of medicine (cardiology) and of immunobiology, could revolutionize our understanding of immune signaling. The study, published May 24 in Nature Communications, reveals the existence of a previously unknown signaling complex, referred to as ZFYVE21-Rubicon-RNF34 (ZRR), which plays a pivotal role in promoting inflammasome activity within endosomes.
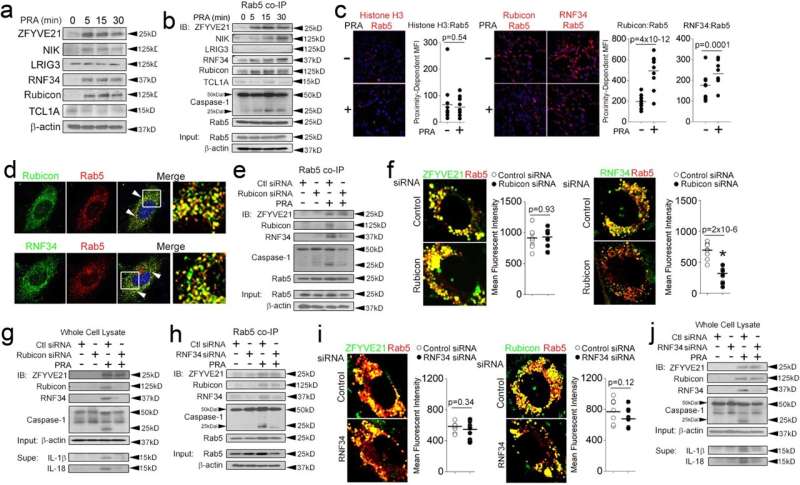
The researchers focused on understanding how inflammasomes are activated in endothelial cells, which are vital for immune function. First authors Xue Li, Quan Jiang, and Guiyu Song used a sophisticated bioinformatics strategy to identify the ZRR complex and its role in facilitating inflammasome activation on endosomes.
They showed that ZFYVE21 recruits Rubicon and RNF34 to endosomes, facilitating the assembly of an active inflammasome complex. Through meticulous laboratory experiments and genetic manipulations, the authors demonstrated that this signaling complex is responsible for activating a protein called caspase-1. This leads to the secretion of pro-inflammatory cytokines, including IL-1β and IL-18, which are vital for the body’s response to inflammation.
This research suggests that unraveling the signaling and trafficking processes within endosomes may reveal druggable targets for modulating inflammasome activity. By uncovering the role of the ZRR signaling complex, the authors have identified a promising target for future therapeutic interventions aimed at controlling inflammation. The implications of this research extend beyond our understanding of immunity, offering hope for improved treatments and better quality of life for individuals living with inflammatory diseases.
More information:
Xue Li et al, A ZFYVE21-Rubicon-RNF34 signaling complex promotes endosome-associated inflammasome activity in endothelial cells, Nature Communications (2023). DOI: 10.1038/s41467-023-38684-2
Journal information:
Nature Communications
Source: Read Full Article