Giant cell arteritis (GCA), formerly known as temporal arteritis, was first described by Mayo Clinic doctors and classified as an inflammatory condition of the blood vessels in the early to mid-20th century. The condition causes bulging blood vessels at the temples, which if left untreated, may lead to blindness, stroke or even an aortic aneurysm. The cause of GCA is unknown.
Nearly 200,000 people in the U.S. are affected. Most are over the age of 50. The disease is more common in Rochester, Minnesota, than other locations due to the area’s large resident populations of Northern European or Scandinavian descent, which may be genetically predisposed to GCA.
Over the last three decades, Cornelia Weyand, M.D., Ph.D., a Mayo rheumatologist and clinician-scientist, has been building on the work of her predecessors to investigate the source of the disease. Her work has illuminated the immune system’s role in the inflammatory process and paved the way for potential new therapies. These insights may also lead to immune therapies that treat more common diseases, including cancer.
Surprising from the start
Bayard Horton, M.D., Thomas Magath, M.D., Ph.D., and George Brown, M.D., first observed the symptoms of GCA—including bulging arteries at the temples—in two different patients and were among the first in the world to perform temporal artery biopsies, publishing their findings in 1934. They also were among the first to classify the condition as a vasculitis, but they didn’t have an explanation for how the disease worked.
A physician in England, J. R. Gilmour, M.B., C.M., renamed the disease giant cell arteritis due to the enlarged cells he observed in 1941. But a significant breakthrough did not come until a decade later, when in 1950, Mayo Clinic physicians Edward Kendall, Ph.D., and Philip Hench, M.D., received the Nobel Prize for the discovery of cortisone. Because cortisone suppresses inflammation, it became one of the primary treatments for GCA. However, the treatment doesn’t get to the source of the condition.
From early on, scientists were surprised to find that GCA involves the immune system. Blood vessels, critical to the body’s function, tend to evade the inflammatory and damaging processes of immune cells, a characteristic that researchers refer to as “immune privilege.” However, GCA targets the blood vessels, including the aorta, the largest artery in the body.
Taking a closer look
Nearly a decade passed before another Mayo physician looked at the disease more closely.
“When I started, no one was interested in giant cell arteritis,” says Gene Hunder, M.D., a rheumatologist and emeritus physician at Mayo.
He says GCA was not on physicians’ radar simply because the disease had not been well defined.
“If you know about something, you see it in a patient and make a note of it. If you aren’t aware of it, you let it go by. I think that was what was happening with giant cell arteritis for many years,” Dr. Hunder says.
He recalls seeing a patient at Mayo in the mid-1960s who presented with a barely palpable pulse and was diagnosed with inflammation of the aorta. He and a resident examined the case in detail. Then, they delved into the medical files to find records of several patients with low pulses in their arms or neck but no further diagnoses. They conducted an epidemiologic study, which provided evidence that patients with GCA frequently also had inflamed aortas.
The finding attracted the attention of the medical world. “I think the fact that the aorta was involved struck the doctors harder because that’s a big blood vessel and could have disastrous side effects,” Dr. Hunder says.
It’s known that GCA is more prevalent in women than men, but no one can predict who will get it. The frontline treatment—the corticosteroid prednisone—is the same approach as half a century ago but relieves symptoms only temporarily.
“In vasculitis, it’s not unusual for some symptoms to go away, but the inflammation in the arteries will go on. The activity of the disease declines with the corticosteroid therapy, but there is still inflammation in the blood vessel,” Dr. Hunder says.
Picking up the thread
In 1990, Dr. Weyand joined Mayo Clinic and soon saw the scientific opportunity to diagnose and treat many patients with GCA in Minnesota and to study the disease.
A few years ago, she and her colleagues identified a problem with a protein that seemed to cause the immune system to go haywire in patients with GCA.
A typical immune system responds to antigens, or toxins, that cause infection or are derived from cancerous cells. While immune protection against infection and cancer is a high priority, the immune system also needs built-in brakes, or immune checkpoints, that tell the immune system to stop and prevent it from over-responding.
Dr. Weyand and colleagues, including Jörg J Goronzy, M.D., Ph.D., found that the protein PD-L1 is weakly expressed in patients with GCA, preventing the delivery of a stop signal to T cells. This finding led the team to conclude that patients with GCA have a broken immune checkpoint, which explains an overabundance of inflammation.
“That became a landmark paper because it was the first report of human autoimmune disease linked to a nonfunctional [immune] checkpoint,” she says.
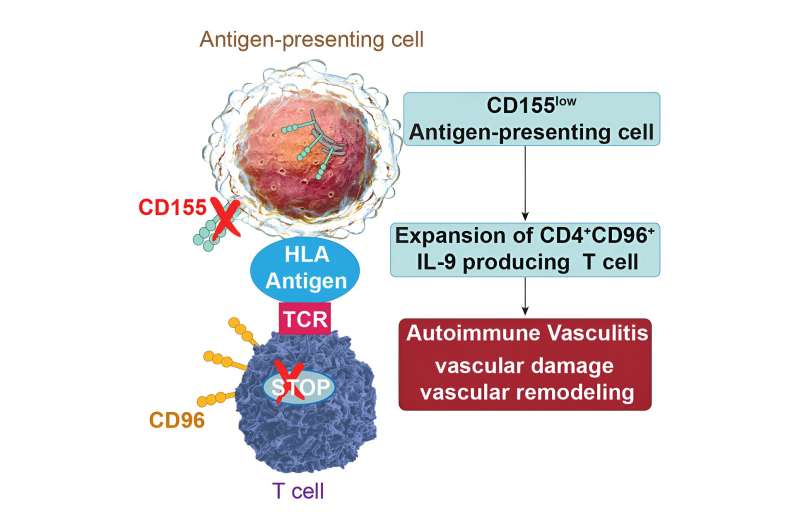
Recently, her research team made another discovery, furthering the understanding of an immune system gone awry. They found a second immune checkpoint that is not working in GCA. They saw that a specific ligand, a molecule that binds to and activates a receptor on immune cells, gets trapped and can’t make it to the surface of the immune cell to signal the immune system to stop.
When the ligand, called CD155, gets trapped, it triggers the unrestricted proliferation of T cells. The T cells invade and accumulate in blood vessel walls. There, they secrete the cytokine interleukin-9, which further attacks and damages the blood vessel.
“We now have seen a second checkpoint that is not working. So, this seems to be the reason why these patients are driving inflammatory responses in the aorta where they are not supposed to be,” Dr. Weyand says.
That study, published in Cell Reports Medicine, shows the immune cells lacking the CD155 ligands also can be detected in blood and GCA lesions and therefore are new biomarkers of the disease that may help improve diagnosis.
What’s more, the researchers have shown in another paper, published in Science Translational Medicine, that the structure of the blood vessel tissues itself shelters and fosters the disease-inducing immune cells in GCA. This provides even more information for potential GCA therapies.
Advancing immunology has relevance to cancer, too
These advances in the immunology of GCA have relevance to cancer, too, Dr. Weyand points out. Increasingly, immune therapies that enhance a person’s own immune response are being used to treat various forms of cancer.
Patients with cancer are on the opposite end of the spectrum from patients with vasculitis in that their immune checkpoints, or brakes, work too well to stop immune response, and tumors take advantage of that. As a result, oncologists prescribe immune checkpoint inhibitors to unleash patients’ immune systems to fight cancer cells and tumors.
Ultimately, knowing how to target faulty immune checkpoints opens the opportunity to identify new GCA treatments. Dr. Weyand’s team has developed a preclinical model outfitted with human arteries and a human immune system called an immune avatar. The model system enables researchers to study GCA more closely, monitor inflammation and test immunotherapies to suppress inflammation.
“That immune avatar has been a dimensional step forward for us,” she says. “This research changes our fundamental understanding of the pathogenesis of giant cell arteritis and paves the way for an entirely new approach for treatment.”
More information:
Shozo Ohtsuki et al, Deficiency of the CD155-CD96 immune checkpoint controls IL-9 production in giant cell arteritis, Cell Reports Medicine (2023). DOI: 10.1016/j.xcrm.2023.101012
Yuki Sato et al, Stem-like CD4 + T cells in perivascular tertiary lymphoid structures sustain autoimmune vasculitis, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.adh0380
Journal information:
Science Translational Medicine
,
Cell Reports Medicine
Source: Read Full Article