Antibodies are crucial, not only for treating tumors and infections. Sometimes, however, the immune reaction they trigger can be too strong and end up causing more damage, for example in the case of people infected with COVID-19. Problems such as these can often be avoided by fine-tuning antibodies, as Prof. Dr. Falk Nimmerjahn from Friedrich–Alexander University and two of his colleagues in the Netherlands and in the UK have reported in the journal Nature Immunology.
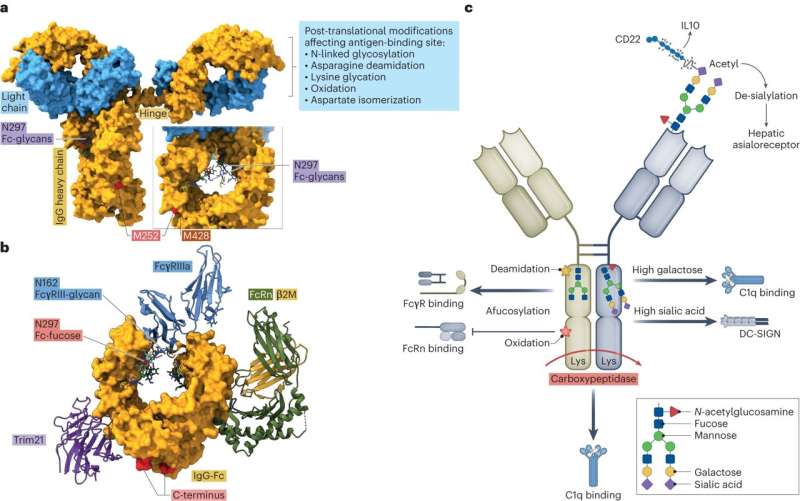
In his laboratories, the FAU researcher is carrying out research into immunoglobulin G, or IgG in short, that provides long-lasting protection against infection in the bodies of humans and animals. These biomolecules that are often used in modern medicine consist of two long and two short chains of proteins that link together to form a Y-shaped structure.
For many years, research and medicine has focused on the two top branches of this Y for good reason: the two ends form a type of pocket which smaller structures on the surface of bacteria and other pathogens fit into, similarly to a key in a lock.
Key-lock principle in immune system
Just like a locksmith can produce very many different locks and the matching keys by only making a few slight changes, the immune system also produces very many different structures at the ends of immunoglobulins that match to very many different pathogens. After an infection with a specific bacterium or virus, these IgG created during the immune reaction remain on patrol within the body for a very long time and can react extremely rapidly in the case of a renewed infection.
If the key fits the lock, the immunoglobulin attaches to the pathogen and marks it for other immune specialists within the immune system. The antibody serves to mark tumor cells or pathogens to make them stand out from the huge quantities of cells and harmless microorganisms that circulate throughout the body and take on important functions in the bodies of humans and animals.
Using genetic glue to fight bacteria
Once this stage has been successfully completed, this is when the backbone of the Y-shaped IgG comes into play. It is this backbone that Falk Nimmerjahn is now investigating closely. Macrophages, killer cells and granulocytes take over in the end phase of the battle against an infection. “We have often observed cells working as a team, with granulocytes taking on a suicidal role,” Falk Nimmerjahn explains.
Attracted by the antibody that has found its target, these cells burst, releasing their relatively sticky genetic material from their core. The bacteria that the IgG previously identified as being harmful stick to this matter.
These microorganisms can be extremely dangerous, but have now been rendered helpless, and are easy prey for the macrophages that have also been attracted and can now consume the bacteria that the antibodies have tracked down and marked. However, the macrophages are often rather aggressive and act with little consideration of possible consequences.
If time is running out in the race between life and death, collateral damage is accepted as being unavoidable, and substances such as oxygen radicals and other dangerous products that would normally be rendered harmless are released. For most patients this is of no consequence. The main priority is survival, any resulting damage should be able to be repaired later.
One of the factors modulating the immune reaction involves small posttranslational modifications that are made to the backbone of the immunoglobulin after the antibody has been created. This involves, for example, little sugar molecules that are attached to the backbone of the immunoglobulin. They seem to play a crucial role in the fine-tuning of the immune reaction. “If the right components are missing, it makes the immune reaction much more severe,” explains Falk Nimmerjahn.
That can, however, have fatal consequences, for example if a viral infection has already severely damaged tissue. If the control mechanism on the backbone of the immunoglobulin is adjusted to only attach a little sugar and therefore induce a strong reaction, that may cause dangerously severe damage to an organ that is already stretched to its limit, such as the lung in the event of a viral infection.
According to Falk Nimmerjahn, “the organism therefore adjusts its control mechanisms very exactly.” In cases such as this, the control mechanisms are set to trigger a weak reaction with many chains of sugar. Gaining an exact knowledge of this antibody tuning within the context of an immune response is fundamental if we are to improve and increase patients’ tolerance of antibodies used to treat tumors and autoimmune diseases.
More information:
Falk Nimmerjahn et al, Effect of posttranslational modifications and subclass on IgG activity: from immunity to immunotherapy, Nature Immunology (2023). DOI: 10.1038/s41590-023-01544-8
Journal information:
Nature Immunology
Source: Read Full Article