While the importance of the triggering receptor expressed on myeloid cells 2 (TREM2) is known in brain microglial function during neurodegenerative diseases, the function of the TREM2 cell receptor/protein is unknown in glioblastoma tumors. In a new report in Science Advances, Rui Sun, and a research team in the departments of neurosurgery, pathology, anesthesiology and developmental biology at the Washington University School of Medicine, in the U.S., found the cell receptor to be highly expressed in myeloid subsets including macrophages and microglia, by using examples of human and mouse glioblastoma tumors.
When the bioengineers induced TREM2 loss-of-function experiments in human macrophages and mouse myeloid cells they increased interferon-γ-induced immunoactivation, proinflammatory polarization and tumoricidal capacity. The team used mouse models of glioblastoma to show how the chronic and acute TREM2 loss-of-function resulted in decreased tumor growth and improved survival. Inhibiting this cell receptor led to increased production of the programmed cell death protein and CD8+ T cells in the tumor microenvironment. The deficiency of TREM2 therefore improved the effectiveness of anti-programmed cell death protein-1 (PD-1) treatment in preclinical experiments as a viable therapeutic strategy for intervention in patients with glioblastoma.
Treatment strategies for glioblastoma tumors
Glioblastoma is a primary malignant brain tumor with short-term survival in patients, despite the availability of a range of aggressive, multimodal therapeutic strategies. Treating the tumor microenvironment is challenging due to its “cold” nature, thereby preventing T-cell infiltration as well as pro-inflammatory responses to render the tumor resistant to immunotherapies such as immune checkpoint blockade.
Myeloid cell populations including macrophages and microglia account for more than 50% leukocyte enriched in the tumor microenvironment, although they rarely exert tumoricidal functions—while maintaining an immunosuppressive environment instead. Oncologists and bioengineers therefore seek to develop new strategies to treat glioblastoma.
Role of triggering receptor expressed on myeloid cells 2 (TREM2)
Neuroscientists have extensively studied the triggering receptor expressed on myeloid cells 2 (TREM2) for its function in microglia in neurodegenerative diseases such as Alzheimer’s disease. Recent studies have shown that specific solid tumors express a high level of TREM2 receptor/protein, although its role in cancer appears to depend on the tumor-type. While the TREM2 receptor/protein suppressed the progression of malignant colorectal cancer and afforded protection from hepatocellular carcinoma, its increased expression in non-small cell lung and gastric tumors resulted in poor prognosis.
Research evidence has shown that the receptor of interest is widely expressed in tumor-associated macrophages, and its loss-of-function altered tumor macrophage behavior within solid tumors of the body for improved immune checkpoint blockade. Nevertheless, much remains to be known of the role of TREM2 during glioblastoma progression including the mechanisms underlying its capacity to regulate myeloid cell function.
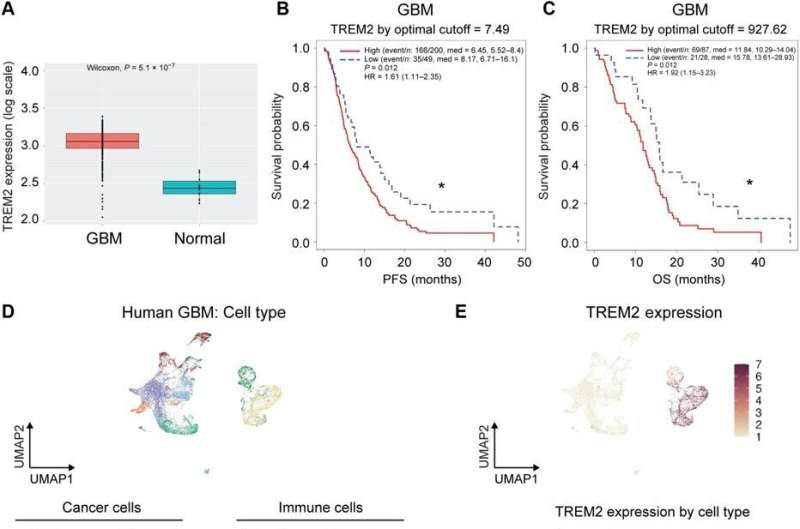
TREM2 expression is higher in glioblastoma tumors when compared to normal brain tissue
Sun and colleagues studied the clinical relevance of TREM2 in human glioblastoma by analyzing if its expression was altered in glioblastoma tumors; typically classified as IDH1/2-wild type tumors in the cancer genome atlas. The mRNA levels of the TREM2 receptor were significantly higher in wild-type glioblastoma tumors, compared to normal brain tissue. In gliomas in general, the expression of the receptor correlated with the cancer grade, where the highest levels were found in grade 4 gliomas. The team studied if the TREM2 levels were associated with clinical outcomes by classifying the glioblastoma tumor type into high/low TREM2 expression categories.
They studied the specific cell types expressing TREM2 in tumor patients, by examining single-cell RNA sequencing datasets and using unsupervised clustering by uniform manifold approximation and projection. They thereby identified immune cell markers and hypothesized the TREM2 expressing tumor-associated myeloid population to play a significant role during glioblastoma tumor progression.
Regulating glioblastoma tumors with myeloid cells
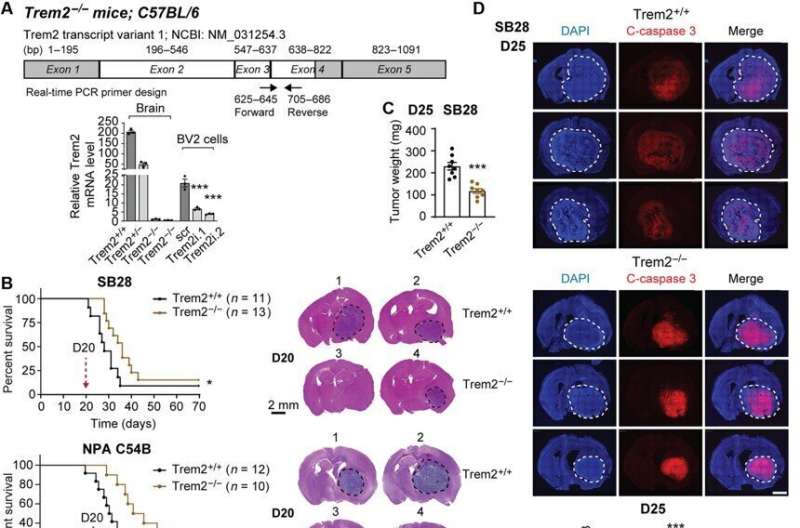
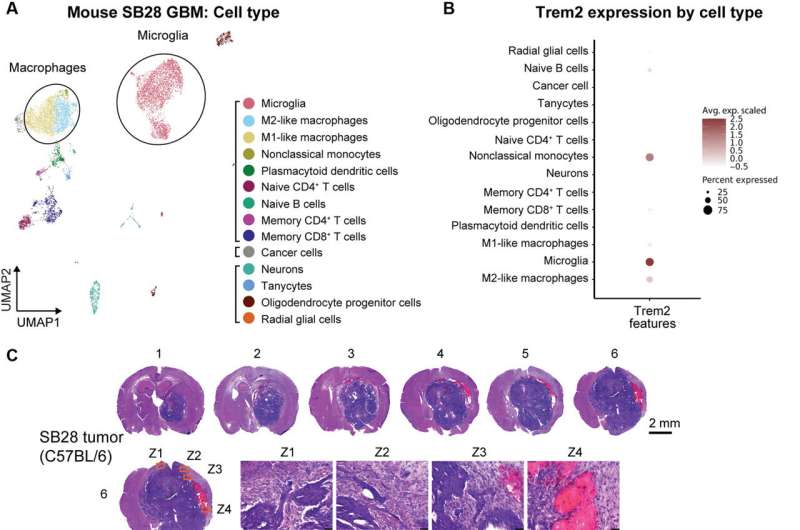
The scientists explored the role of TREM2 by using glioblastoma mouse models recapitulating characteristics of human glioblastoma, including resistance to radiotherapy and chemotherapy, resistance to immune checkpoint blockade, alongside a high fraction of myeloid cells in the tumor microenvironment. As with human tumors, single-cell RNA sequence results revealed predominant macrophage/monocyte lineages and microglial cells.
The microglia showed markedly high levels of Trem2 expression among a range of anti-inflammatory cell lines. They noted M1-like interferon-γ induced cells to maintain relatively low-levels of TREM2 expression. The team used transgenic mice deficient to the cell receptor (Trem2-/-) and noticed more apoptotic cells in such tumors, indicating that its deficiency inhibited tumor growth by increasing tumor cell death.
Additional TREM2 loss-of-function experiments in mouse and human glioblastoma models
The scientists conducted a series of experiments to dissect the role of myeloid cells during glioblastoma progression by using bone-marrow derived macrophages from transgenic mice deficient to TREM2 expression. The results showed increased phagocytic function and cytotoxicity towards the glioblastoma cell line. When they tested TREM2 knockdown in human macrophages, they noted an inclination for enhanced tumor cell death in patient-derived glioblastoma stem cells. The researchers further tested and validated the human model system of patient-derived glioblastoma stem cells in the lab.
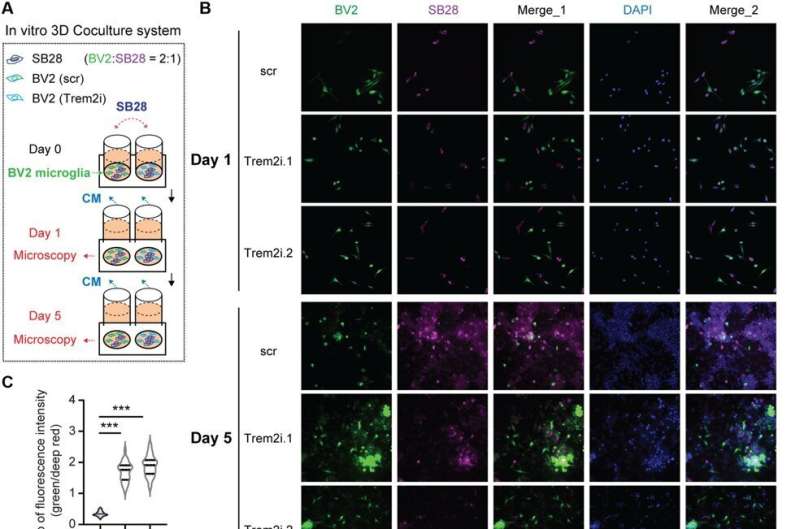
When they used microglia, i.e., brain resident macrophages distinct from those arising from the blood lineage, they noted TREM2 deficiency increased phagocytic function, tumoricidal activity and a pro-inflammatory phenotype in mouse models. The myeloid cells with loss-of-function showed anti-tumor effects both in vitro and in vivo, while reprogramming tumor-associated myeloid populations towards a pro-inflammatory phenotype. Additional observations showed TREM2 deficiency to increase T cells in the tumor microenvironment, while combining the programmed cell death protein for therapeutic strategies and improved animal survival. The deficiency of TREM2 further improved the effectiveness of anti-programmed cell death protein-1 (PD-1) treatment in preclinical experiments.
Outlook
In this way, Rui Sun and colleagues noted higher expression of TREM2 in glioblastoma tumors associated with patients experiencing worse survival rates. They noted increased TREM2 expression in myeloid cells populations within tumor microenvironments, including microglia and macrophage subsets.
The team carried out extensive experiments in vitro and in vivo in human and mouse model systems to show effects of TREM2 inhibition on tumor growth reduction, with the promise to formulate additional therapeutic opportunities in the future.
More information:
Rui Sun et al, TREM2 inhibition triggers antitumor cell activity of myeloid cells in glioblastoma, Science Advances (2023). DOI: 10.1126/sciadv.ade3559
Cyril Neftel et al, An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma, Cell (2019). DOI: 10.1016/j.cell.2019.06.024
Journal information:
Science Advances
,
Cell
Source: Read Full Article