Transplantation of photoreceptor cells is a promising intervention that in the future could help recover vision in people with blinding diseases. A team of researchers led by Prof. Marius Ader from the Center for Regenerative Therapies Dresden (CRTD) at TU Dresden has just reported another pre-clinical advance in a new study published in the Journal of Clinical Investigation. The team developed a robust method to produce high numbers of human photoreceptor cells. The researchers show that such human photoreceptors can incorporate in bulk into partially degenerated mouse retinas. The incorporated photoreceptors developed characteristics of normal photoreceptors and allowed mice with damaged eyesight to detect daylight.
The new study represents a step forward in an effort to bring photoreceptor transplantations to patients with blinding diseases. “To our knowledge, this is the first time someone has achieved such a massive integration of transplanted photoreceptors into the retina,” says Prof. Marius Ader, research group leader at the Center for Regenerative Therapies Dresden (CRTD) at TU Dresden who led the study.
Multiple factors at play
To massively increase the number of incorporated photoreceptors, the scientists optimized multiple critical factors. They established that the age of transplanted photoreceptors is decisive. “We have managed to find what seems to be a perfect stage for transferring the photoreceptors into the retina. If we do it with younger or older photoreceptors, we see a sharp decline in the rate of incorporation,” says Prof. Ader.
The team also found that the integration into the retina needs a longer time. “We have seen that the photoreceptor cells need substantial time, up to six months, to establish interactions and build a proper network with the remaining cells in the mouse retina,” says Prof. Ader.
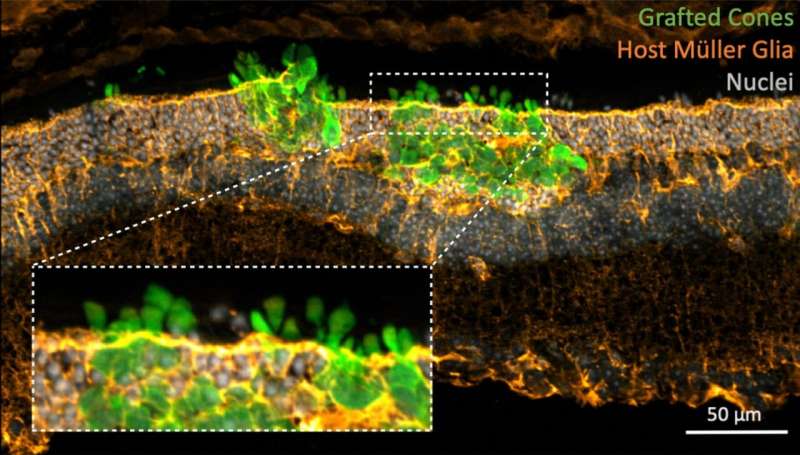
The interaction with the remaining, undamaged cells in the mouse retina turned out to be a key factor. “About 30% of the cells in the retina are other cells that support the work of photoreceptors. In our case, we clearly saw that the interaction of transplanted cells with host retinal cells was crucial for successful incorporation and maturation. Some of these remaining cells provided a scaffold for the new photoreceptors and helped them organize correctly,” adds Prof. Ader.
Robust and unlimited source of photoreceptors
To produce photoreceptors, the team used stem cells to grow mini-retinas in a laboratory dish, according to a protocol developed by their collaborator Prof. Mike Karl. Once the mini-retinas were developed to the appropriate stage, Dr. Sylvia Gasparini and Karen Tessmer, scientists in Prof. Ader’s team who performed most of the experiments in this study, harvested the photoreceptors for transplantation.
“Obtaining a pure population of photoreceptors is yet another challenge. To address it, our collaborator Prof. Volker Busskamp developed a new stem cell line in which cone photoreceptor cells have special tags. These tags do not interfere with their function but allow us to robustly sort photoreceptors from the rest of the cells in the mini-retinas,” explains Prof. Ader.
Such induced pluripotent stem cell lines provide a virtually unlimited source of photoreceptors and can potentially be used in future clinical applications.
Restoring daylight perception
In this study, the team focused on mice with partially degenerated retinas that lacked only one out of two types of photoreceptors. “The mice had only damaged cones, which are responsible for daylight vision, a situation similar to several blinding diseases in human patients,” explains Prof. Ader. The approach was different from previous studies because the remaining cells in the retina were undamaged. So far, most of the transplantation attempts targeted models of very late-stage blinding diseases, characterized by degeneration of all photoreceptors.
Together with their collaborators at the Natural and Medical Sciences Institute in Tübingen, University of Bonn, German Center for Neurodegenerative Diseases (DZNE) Dresden, DRESDEN-concept Genome Center, and electron and light microscopy facilities at the Center for Molecular and Cellular Bioengineering (CMCB) of TU Dresden, the team has employed a variety of techniques to thoroughly validate the maturation and function of transplanted photoreceptors. They were able to demonstrate that the new photoreceptors not only adopt physiological characteristics of normal photoreceptors but also, as shown by their collaborator Prof. Günther Zeck, function properly providing signals to nerve cells downstream in the retina.
Source: Read Full Article