The ongoing global pandemic of coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pathogen. This virus has infected more than 128 million individuals and has claimed about 2.8 million lives.
Several COVID-19 vaccines have been approved by various regulatory bodies and are currently being rolled out across many parts of the world. There are more than 200 COVID-19 vaccines under development, and 70 are undergoing clinical trials. Several strategies have been employed in the development of different vaccine models, including adenovirus viral vectors (the Oxford-AstraZeneca model); lipid nanoparticles encasing viral mRNA (Moderna, Pfizer/BioNTech); inactivated DNA viruses (Sinopharm, Sinovac, Janssen), viral subunits, and live attenuated viruses. Despite being effective, some strategies contain limitations.
.jpg)
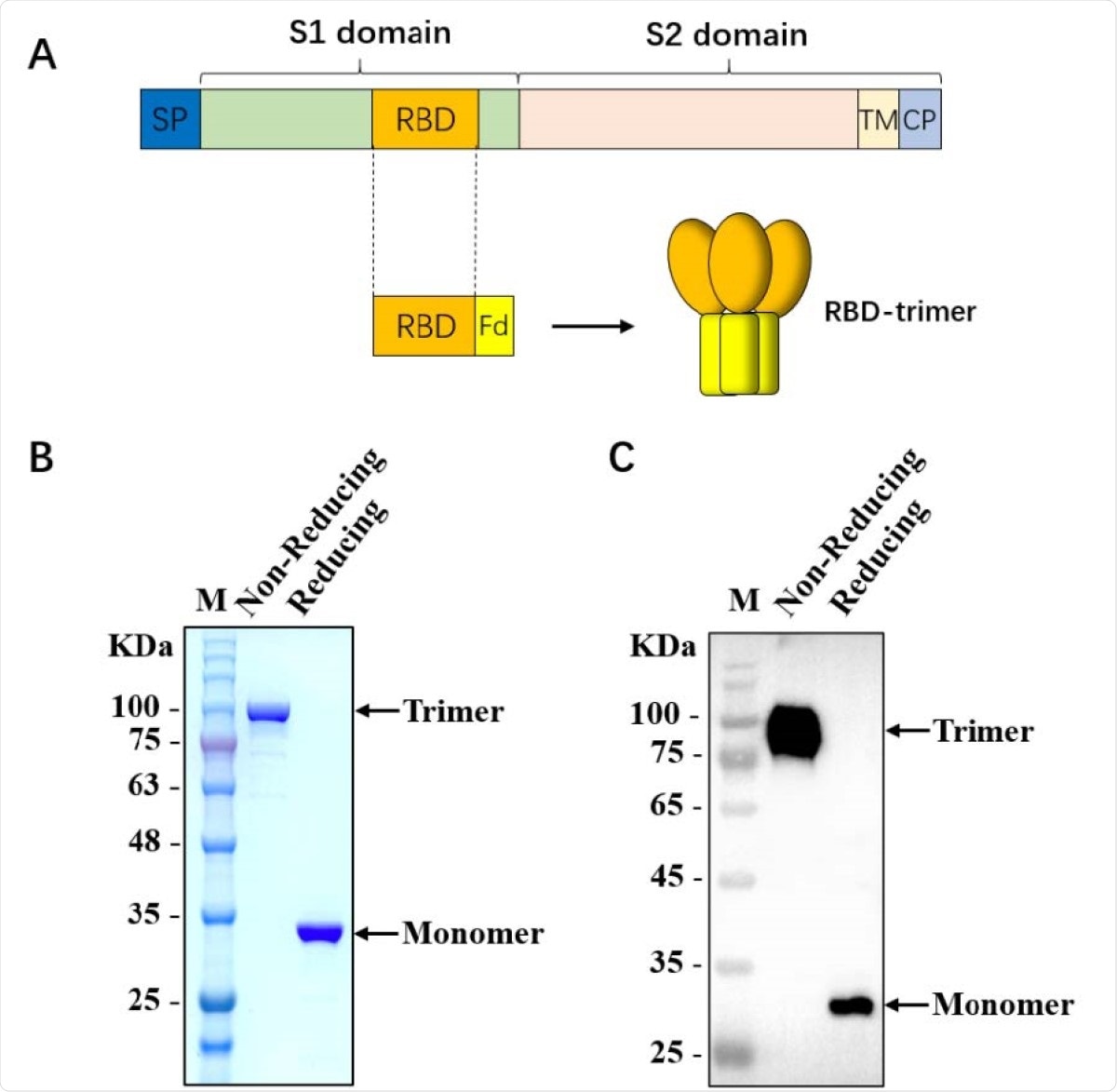
SARS-CoV-2 is an RNA betacoronavirus that belongs to the family Coronaviridae. Other viruses of the same family that can infect human populations are the Middle East Respiratory Syndrome (MERS) and SARS-CoV. This virus infects the host cells via its spike (S) glycoprotein, which has become the prime target in vaccine development. Scientists have developed an artificially designed S trimer, mimicking the native S trimer structure. In the construction of an artificial S trimer, the C-terminal region of human type Iα collagen has been merged and effectively used in vaccine research.
Earlier studies have shown that both SARS-CoV and MERS-CoV exhibit antibody-dependent enhancement (ADE), which increases the risk of vaccine-induced susceptibility to the disease. Such a phenomenon occurs when non-neutralizing antibodies are produced in response to the vaccine-mediated virus infection via the Fcγ receptor. Owing to the high degree of similarity between SARS-CoV-2, SARS-CoV, and MERS-CoV, the potential risk of the ADE effect should be addressed while designing COVID-19 vaccines. The ADE effect can be reduced by a) decreasing non-neutralizing epitopes and b) making only critical neutralizing epitopes available for inducing strong immunity.
Scientists have targeted the receptor-binding domain (RBD), which is located at the C-terminus of the S1 subunit, for developing vaccines. This is because previous research revealed that RBD-specific antibodies could minimize the ADE effect. Additionally, most research indicates that the potent neutralizing antibody (NAb) targets the RBD region.
While studying the infection mechanism, researchers found that SARS-CoV-2 initiates its replication process within the host by binding through the RBD to the cell surface receptor angiotensin-converting enzyme 2 (ACE2). Thereby, RBD is an effective vaccine target to promote inhibition of viral binding to the host cells. Further, as RBD possesses T cell epitopes, it can also elicit antiviral T cell responses.
Despite all the positivity of RBD, it promotes weak immunogenicity due to its small molecular weight. Thereby, to improve immunogenicity, several researchers prepared a dimer by merging RBD with the Fc domain. The reason why some of the individuals are reinfected with COVID-19 disease is that the NAb level in SARS-CoV-2-infected individuals considerably decreases from the second-month post recovery and eventually wanes off. Thereby, two of the most prominent challenges that researchers face while developing the COVID-19 vaccines are (a) short-lived antibody protection and (b) high mutation rate of SARS-CoV-2. Recently, the efficacy of licensed mRNA vaccines was found to decrease against the 501Y.V2 variant, which has emerged in South Africa. These reports further indicate the need to develop a robust vaccine strategy to produce more efficient and persistent vaccines that would protect against the variants.
A new study released on the bioRxiv* preprint server deals with the development of RBD trimer as a potent target for SARS-CoV-2 subunit vaccines. This study revealed the development of a trimeric RBD subunit vaccine candidate that could induce strong humoral and cellular immune responses in nonhuman primates (NHPs), namely, rhesus macaques.
This vaccine candidate not only elicited a robust immune response by producing a high titer of NAbs but also developed potent CD4 and CD8 T cell immune responses. The immune protection was reported to last for at least four months. Additionally, the vaccinated individual could rapidly realize immune protection as the booster immunization could instantaneously stimulate the memory immune response. The vaccine-induced antibodies also revealed prominent neutralizing activity against the 501Y.V2 variant. Further, qPCR and immunohistochemical assays were used to study the presence of virus in the lungs of the non-primates. The results indicated the absence of viral RNA in the lungs of the vaccinated non-human primate. Besides the lungs, SARS-CoV-2 can also damage other host organs such as the liver. In this study, researchers found that the vaccine candidate effectively protects the organs of NHPs from virus attacks.
In this research, scientists have fused the trimer motif to develop a more stable trimer structure. This ensured improved immunogenicity of the recombinant RBD protein. It also mimics the native trimeric SARS-CoV-2 S structure. The newly developed structure was found to be stable at a temperature of above 60℃. Further, no change was observed even after 3 months of storage at 4℃.
The data obtained after vaccination revealed that the newly developed RBD-based subunit vaccine showed no abnormalities in the vaccinated primates, in regards to weight, body temperature, clinical signs, pathology, hematology, and biochemical indicators. Thereby, scientists are highly optimistic that this trimeric RBD can be a promising vaccine candidate against COVID-19.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Limin Yang et al. (2021) A recombinant receptor-binding domain in trimeric form generates completely protective immunity against SARS-CoV-2 infection in nonhuman primates. bioRxiv 2021.03.30.437647; doi: https://doi.org/10.1101/2021.03.30.437647, https://www.biorxiv.org/content/10.1101/2021.03.30.437647v1
Posted in: Medical Science News | Medical Research News | Disease/Infection News | Healthcare News
Tags: ACE2, Adenovirus, Angiotensin, Angiotensin-Converting Enzyme 2, Antibodies, Antibody, CD4, Cell, Collagen, Coronavirus, Coronavirus Disease COVID-19, DNA, Efficacy, Enzyme, Glycoprotein, Hematology, Immune Response, Immunization, Liver, Lungs, MERS-CoV, Mutation, Nanoparticles, Pandemic, Pathogen, Pathology, Protein, Receptor, Research, Respiratory, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine, Virus

Written by
Dr. Priyom Bose
Priyom holds a Ph.D. in Plant Biology and Biotechnology from the University of Madras, India. She is an active researcher and an experienced science writer. Priyom has also co-authored several original research articles that have been published in reputed peer-reviewed journals. She is also an avid reader and an amateur photographer.
Source: Read Full Article