A research team led by the German Cancer Research Center in Heidelberg, Germany, has discovered that the genetic sequence of a tumor can be read like a molecular clock, traced back to its most recent common ancestor cell. Extracting the duration of tumor evolution can give an accurate predictor of neuroblastoma outcomes.
In a paper published in Nature Genetics titled “Neuroblastoma arises in early fetal development and its evolutionary duration predicts outcome,” the team details the steps they took in identifying a genomic clock tested against a whole genome sequenced population combined with analysis and mathematical modeling, to identify evolution markers, traceability and a likely origin point of infant neuroblastomas.
Cancer cells start out life as heroic healthy tissues, with the sort of all for one, one for all, throw yourself on a grenade to save your mates–type attitude that is taking place throughout the body every day. At some point, something goes wrong, and a good cell goes bad.
It begins with miscommunication in dividing or an injury to a cell’s DNA, which happens with great regularity throughout the body. Typically this is handled before trouble can start by a repair mechanism. If the repair cannot be made, it is time to engage in apoptosis or cell death.
A healthy cell response to a call for apoptosis is to throw itself on the “grenade” to keep all of the surrounding cells and cell tissues safe. If a cell is not able to hear the call for apoptosis, as can happen with chromosomal damage, it takes no action. Without the conforming behavior to grow or stop growing, a cell becomes a threat to its surroundings, a rogue cell, a cancer cell and—if it proliferates unchecked—a tumor.
A cell on its own, no longer able to communicate with its neighbors because the communication network between cells has been lost, still tries to survive. Within a cell is most of what it needs to continue the mission of life—to grow, to reproduce, and to thrive. But from the outside the perspective is very different. From outside the isolated and injured cell, cancer is growing. Depending on where the cancer is, and how it is constructed, the outcomes can be very different.
In the case of neuroblastoma, the most frequent solid tumor in infants, a wide spectrum of clinical outcomes is possible, ranging from low-risk cases requiring light or no treatment to a high-risk situation that is fatal for about 50% of patients.
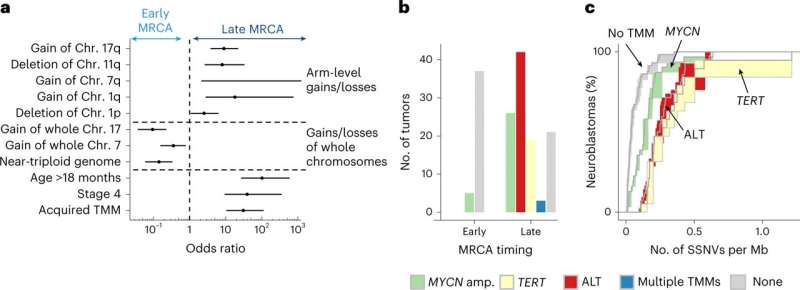
In the current study, cohorts of primary and relapsed neuroblastoma tumors were retrospectively analyzed. Whole genome sequencing was applied to 100 neuroblastomas and validated in an independent group of 86. Neutral single-nucleotide variants were selected and tracked as a molecular clock to time key events.
By comparing tumor clone sequences to the molecular clock, researchers found that the density of somatic single-nucleotide variants (SSNVs) in cloned cells was similar for the different copy numbers, essentially a continuation of the molecular clock in the clone, and as a result, traceable back to a most recent common ancestor cell. Compare this to standard tumor sampling that can tell if different tumor cells are related but lacks the time-dependent evolutionary connection, and the importance of this method becomes strikingly clear.
Researchers further discovered that the duration of evolution was found to be an accurate predictor of outcome. Cells that quickly became tumors did so without the ability to sustain growth, while others that more slowly transitioned into tumors built an infrastructure for more prolonged and aggressive tactics for survival. Knowing this, researchers could then identify neuroblastomas with favorable clinical outcomes.
Origin story
Researchers were able to wind back the molecular clock of their model and pinpoint a likely origin of neuroblastomas by combining whole-genome sequencing, molecular clock analysis and population-genetic modeling in a comprehensive cohort covering all subtypes. Tumors across the entire clinical spectrum likely begin to develop via aberrant mitoses as early as the first trimester of pregnancy. This is when the adrenal medulla forms from sympathetic neuroblasts, and the modeling suggests that the initial oncogenic event is limited to this time window. The transcriptomes of neuroblastomas most resemble those of sympathetic neuroblasts that are highly proliferative in the first trimester, which may make them vulnerable to aneuploidy (chromosomal abnormalities).
A tremendous effort is underway now to compile a gene atlas, a document of every cell in the human body, their functions and interconnected communications. Everything that science has revealed, from basic anatomy to the discovery of DNA and the human genome project. From unraveling the ways that DNA is transcribed, spliced, translated, and modified to how epigenetics and gene variants affect health. All of it leads to a platform from which we can understand molecular cellular evolution in predictive enough ways to take proactive, preventative and procedural steps to ensure sustained longevity of human life.
The current study is a window into that future, with a predictive and time-dependent understanding of the molecular evolution of a single tumor cell type. The age of modern miracle medical science is not the age we are living in, but it is the age we are now building.
More information:
Verena Körber et al, Neuroblastoma arises in early fetal development and its evolutionary duration predicts outcome, Nature Genetics (2023). DOI: 10.1038/s41588-023-01332-y
Giulio Caravagna, Mathematical modeling of neuroblastoma associates evolutionary patterns with outcomes, Nature Genetics (2023). DOI: 10.1038/s41588-023-01358-2
Journal information:
Nature Genetics
Source: Read Full Article