Acute kidney injury (AKI) is a frequent complication associated with various diseases and particularly affects patients on intensive care units. However, the mechanisms underlying AKI are incompletely understood. Just recently, an interdisciplinary research team has used single-cell sequencing techniques to uncover the molecular processes associated with AKI.
Reporting in Genome Medicine and Kidney International, they describe novel gene expression patterns of injured kidney cells that may lead to new therapeutic approaches and strategies for biomarker discovery. The studies were conducted in close collaboration between Charité—Universitätsmedizin Berlin; the Berlin Institute for Medical Systems Biology (BIMSB) of the Max Delbrück Center; the German Rheumatism Research Center Berlin (DRFZ), a Leibniz Institute; and the Hannover Medical School.
The kidneys are among the most important organs in the human body. They filter waste products from the blood, control body fluid composition and blood pressure, influence energy metabolism, and produce vital hormones. If kidney function is impaired—as is the case in AKI—there can be severe consequences.
“AKI is a frequent and serious complication in critically ill patients, affecting about half of our intensive care unit patients,” says Dr. Jan Klocke of Charité’s Department of Nephrology and Medical Intensive Care. “The condition is often underestimated, despite the fact that AKI is associated with increased mortality and patients can suffer permanent damage, even complete loss of kidney function.”
AKI can accompany a wide range of diseases. It often occurs in conjunction with cardiovascular diseases or severe infectious diseases such as COVID-19, but also after surgical interventions or in association with drug treatment. There are often no concrete treatment options.
“We try to stabilize affected patients, but so far it is usually not possible to reverse the destructive processes in the kidney with targeted treatments,” says Dr. Hinze, who played a key role in supervising one of the studies at Charité and the Max Delbrück Center and now works at the Hannover Medical School.
“Up to now, little has been known about which mechanisms are at play in the kidney cells. The aim of our studies was to shed some light on this, with the long-term goal of improving the treatment provided to our patients in the clinic.”
AKI is often triggered by an insufficient supply of blood to the kidneys, causing the cells there to no longer receive sufficient oxygen and nutrients—and to react with stress. The cells go into a kind of alarm mode and produce signal substances that can lead to inflammatory and remodeling processes (fibrosis) in the surrounding tissue.
It is known from animal model studies that epithelial cells—the cells that line the fine renal tubules—are involved in these inflammatory and fibrotic processes. This was demonstrated using a novel state-of the-art method called single-cell sequencing, which enables researchers to create a detailed profile of the molecular gene expression profiles of thousands of individual cells. But what happens on the cellular level in human AKI?
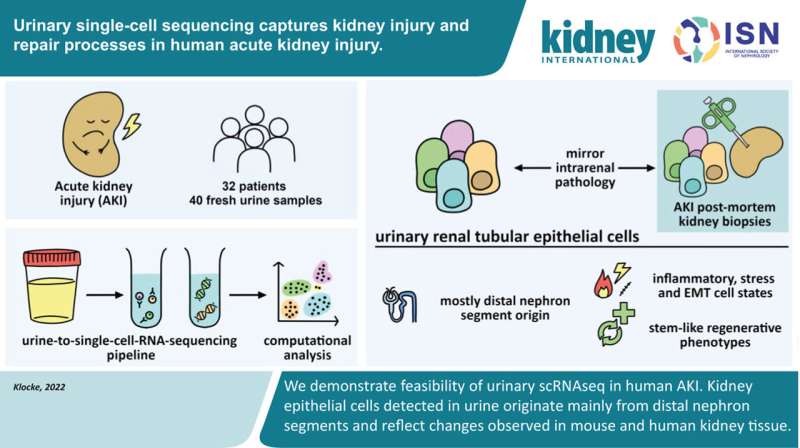
This is the question that research teams led by Dr. Hinze and Dr. Klocke set out to investigate. The two recently published studies are among the first to ever investigate the molecular processes in AKI using single-cell technologies in human kidney cells. The scientists examined cells taken from tissue and urine samples of more than 40 patients and analyzed the molecular patterns of more than 140,000 cells using state-of-the-art bioinformatics approaches.
“Single-cell sequencing allows us to virtually zoom into each cell and see which genes are active in that cell at that point in time,” explains Dr. Hinze. “From this, we can determine whether that particular kidney cell is currently functioning normally, is under stress, or is about to die. This cutting-edge technology gives us an understanding of AKI in unprecedented detail.”
The team was also able to show that different cell types of the kidney react quite differently to AKI, with the strongest response observed in the epithelial cells of the renal tubules. These are the smallest functional units of the kidney and consist of several segments. It was known from animal models that epithelial cells of a specific early renal tubule segment were mainly affected by AKI.
However, the results of the latest studies on human kidney cells have revealed that the epithelial cells of almost all tubule segments are involved in the injury processes.
“This illustrates once again how important it is that we study human systems and learn to understand them better,” says Dr. Hinze. “In the different types of epithelial cells, we were able to identify certain molecular patterns that occurred in all patients with AKI, but at individual abundances. In the future, these findings could help doctors to better assess the risk for severe disease progression.”
In clinical practice, physicians ideally need a fast, non-invasive, and precise testing method to clearly diagnose AKI at an early stage. In order to get closer to this vision for the future, Dr. Klocke started searching for epithelial cells in urine samples. Hardly any cells are found in the urine of healthy people.
But in those with AKI, epithelial cells detach from the renal tubule and are excreted into the urine. However, since cells do not survive in urine for long, there were initial doubts as to whether the cells would still be intact and whether their molecular state could even be measured using single-cell sequencing.
“We processed the urine samples within four to six hours, and it actually worked very well,” says Dr. Klocke. The researchers were able to determine from which segment of the renal tubules the cells came from and which genetic programs they had activated in response to kidney damage.
“The information provided by the cells from the urine samples matched that of the corresponding cells from tissue samples,” says Dr. Klocke. “Thus, urine provides us with an uncomplicated and patient-friendly method of obtaining sample material for further investigations—in order to identify biomarkers and, in the long term, perhaps reduce or even replace kidney biopsies.”
Source: Read Full Article