A multi-institutional research group has succeeded in developing an exon-skipping therapy using nucleic-acid therapeutics for Alport syndrome, an incurable kidney disease that can progress to renal failure. Disease model mice were found to be completely responsive to the treatment. The results were published in Nature Communications on June 2, 2020.
Alport syndrome (AS) is the second most commonly occurring hereditary kidney disease after autosomal dominant polycystic kidney disease (ADPKD). There is one case of Alport syndrome in every 5,000 to 10,000 people. It is characterized by hearing loss, eye abnormalities and kidney disease, often progressing to end stage kidney disease (ESKD). Alport syndrome is divided into three groups according to how it is inherited; X-linked AS, autosomal recessive AS and autosomal dominant AS with approximately 80% of cases being X-linked Alport syndrome (XLAS). Although male patients with XLAS are likely to have severe symptoms and around 90% of them experience end stage renal failure by the age of 40, there is still no specialized treatment for Alport syndrome. XLAS develops due to mutations in the COL4A5 gene that encodes the type IV collagen α5 chain and there is no expression of α5(IV) proteins in the kidneys of male patients with this form of the disease.
Male XLAS patients with severe mutations (such as nonsense mutations) in the COL4A5 gene develop renal failure over 15 years earlier than those with minor mutations (such as missense mutations). In other words, it is thought that if severe mutations could be changed to minor mutations, this would make the symptoms of the disease milder.
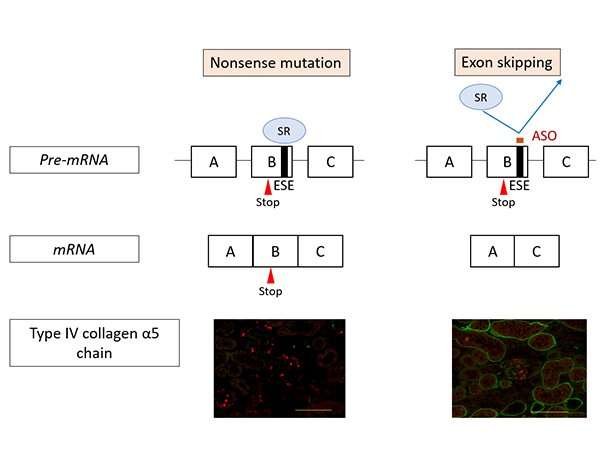
The research team set about developing an exon-skipping therapy using nucleic acid therapeutics (antisense oligonucleotides/ASO) to change severe mutations into minor ones (Figure 1). ASO therapies have received increasing attention in recent years and they have been used to successfully treat intractable inherited diseases such as spinal muscular atrophy and Duchenne muscular dystrophy.
α5(IV) are the most important proteins for the formation of each kidney’s glomerular basement membrane. In XLAS, there is a mutation in the COL4A5 gene that encodes this protein, thus resulting in abnormal α5(IV) production. Severe mutations in COL4A5 (such as nonsense mutations) in particular can lead to ESKD development when the patient is in their early 20s.
However, previous research by Project Professor Nozu et al. has shown that renal failure was delayed by ten years when the number of base deletions was a multiple of three (in-frame mutation). This prompted the researchers to investigate the possibility of reducing symptoms using ASO-mediated exon-skipping therapy to change severe mutations to minor mutations (in-frame mutation).
The majority of exons that make up COL4A5, the casual gene for Alport syndrome, have a number of bases that are a multiple of 3. Therefore, if there is a nonsense mutation, it is possible to replace it with an in-frame (multiple of 3) mutation by deleting the mutated exons during splicing.
Figure 1 shows the action mechanism of exon-skipping by ASO. In nonsense mutation cases, the mutation remains in the messenger RNA (mRNA) resulting in the production of defective proteins. Consequently, α5(IV) proteins are not expressed in the kidneys (as shown on the left in Figure 1, the green signal that indicates the presence of α5(IV) proteins isn’t visible). On the other hand, under ASO-mediated exon-skipping therapy, ASO binds to the Exonic Splicing Enhancer (ESE) sites, blocking the binding of SR proteins to exons, at the pre-mRNA stage. SR protein binding is an important process for exon recognition and if the process is blocked that means that exon skipping can be induced. Consequently, mRNA was generated (bottom right of Figure 1) without a nonsense mutation. When this therapy was tested on model mice with nonsense mutations in COL4A5, α5(IV) protein expression was detected in the kidneys (Green signal in the bottom right of Figure 1).
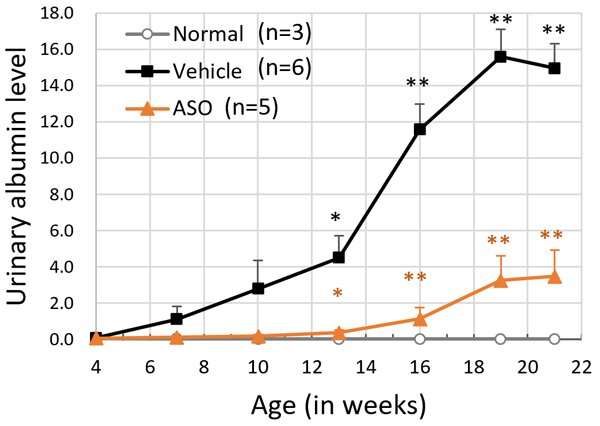
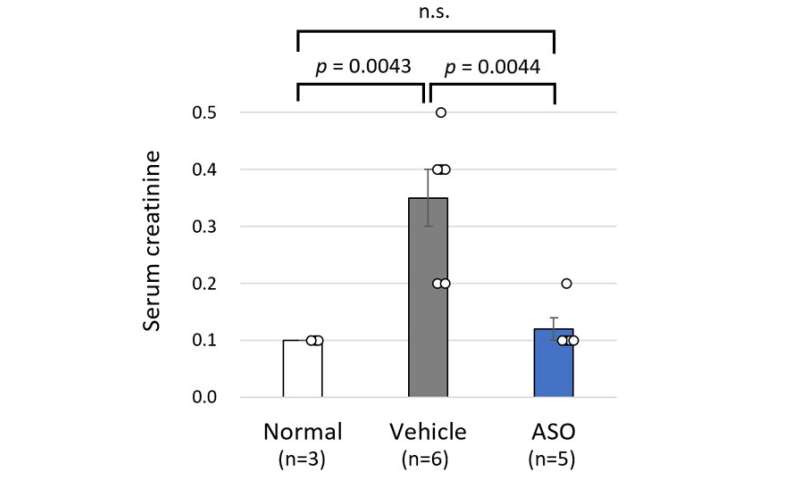
The results for the ASO-treated group and the vehicle treated group of model mice were compared. The mice receiving ASO treatment had lower urinary protein levels than the vehicle group and kidney failure (based on the level of serum creatinine) was suppressed (Figure 3). The researchers also succeeded in significantly prolonging life expectancy with this therapy (Figure 4). In addition to these results, microscopic examination of the kidney tissue indicated that they were able to significantly suppress the progression of renal damage.
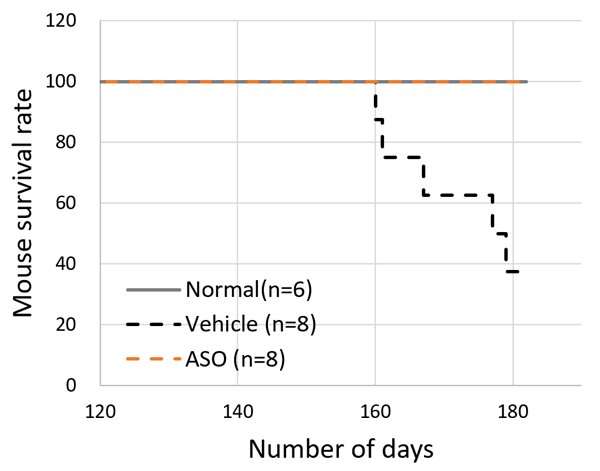
The above results indicated that Alport syndrome model mice with severe genetic mutations were completely responsive to exon-skipping therapy.
Source: Read Full Article