Why do some people develop type 1 diabetes and others do not? Worldwide, researchers are now collaborating to find the answer to this complex question. Diabetes researchers at Lund University recently contributed data to a new study that shows that type 1 diabetes develops in three different ways in children. This improved understanding makes it possible for scientists to conduct new types of studies with the goal of preventing the disease.
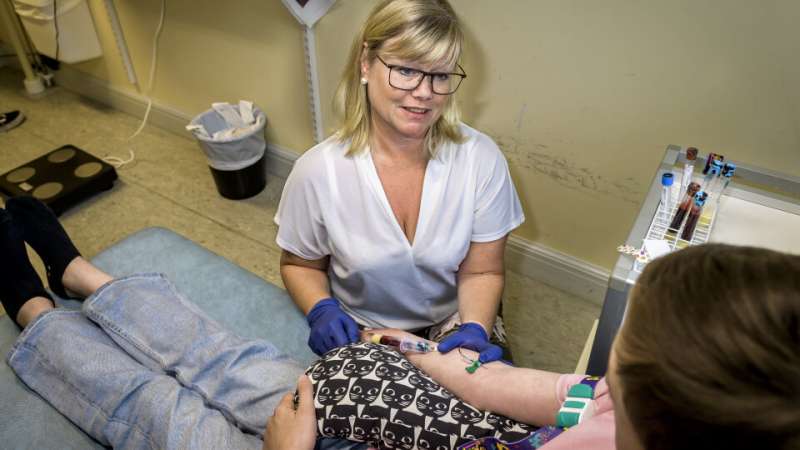
In the TEDDY study in Sweden, every drop of blood counts. Children who participate in the study have an increased genetic risk of developing type 1 diabetes. The first blood samples are taken when the children are four months old, and the testing continues until they reach the age of 15. The children meet the same research nurse during each visit.
“Our nurses are very good at making the visits as comfortable as possible. Sometimes, I feel bad about taking so many blood samples from the children, but it has improved our understanding of why some children develop the disease,” says Åke Lernmark, senior professor in experimental diabetes research at Lund University and principal investigator for the TEDDY study.
Diabetes-related autoantibodies
The study team is particularly interested in following the development of three different diabetes-related autoantibodies in children. With every new study, scientists learn more about these autoantibodies that target the body’s own tissue. A blood sample that shows the presence of one of these autoantibodies indicates that the insulin-producing cells in the pancreas are being attacked by the body’s immune system. It has been shown that an individual with two to four diabetes-related autoantibodies has an increased risk of developing type 1 diabetes.
Lernmark has been interested in this area of research for decades and has helped develop the methods for how blood samples are analyzed.
“It is crucial that we learn more about type 1 diabetes. It is a dreadful disease that can cause a lot of anxiety for those affected by it. Many patients develop the disease at a young age, and long-term it can cause serious complications that in turn decrease quality of life and life expectancy.”
Lernmark is one of the authors of an international study recently published in Nature Communications which shows that type 1 diabetes develops in three different ways in children. The study used data from 24,662 children who were followed for 15 years. Data has been gathered in cohort studies in the U.S., Sweden, Germany, and Finland. Lund University has contributed with data collected in a cohort study in Skåne, Sweden, in which children were screened for diabetes risk from birth until the age of 15. The article shows that type 1 diabetes can be divided into three different groups, depending on what the pattern for developing autoantibodies looks like.
Markus Lundgren, researcher in pediatric endocrinology at Lund University, is principal investigator for the follow-up study in Sweden, and co-authored the study published in Nature Communications.
“This study clearly shows that there are three different routes from healthy individual to disease debut in the children. Our research is strengthened by the fact that several international researchers have contributed data for the analyses that have been done using advanced machine learning,” says Markus Lundgren, who is also a pediatrician specializing in diabetes and endocrine disorders.
Prevention studies
Markus Lundgren and Lernmark are part of an international consortium that aims to use autoantibodies in clinical trials to prevent type 1 diabetes. The European Medicines Agency recently approved the use of diabetes-related autoantibodies as biomarkers, and results from the TEDDY study were used to support the approval. This approval means that individuals who have at least two of these autoantibodies can participate in clinical studies designed to prevent the disease. Until now, it has only been possible to treat study participants who have already developed type 1 diabetes.
“The approval marks a great success for all of us who work to improve the understanding of how type 1 diabetes develops. It illustrates how important international collaborations are in achieving results. The children who have taken part in our studies have indeed made very meaningful contributions. We hope that the approval will make pharmaceutical companies interested in developing new drugs and treatments that can prevent the development of type 1 diabetes in children with autoantibodies,” says Lernmark.
Markus Lundgren leads a study in Skåne in which researchers are investigating whether a new drug can preserve insulin-producing cells in adults who have recently developed type 1 diabetes. The study forms part of an international research collaboration. He hopes that similar studies can be conducted for preventive purposes in the future.
“If the drug that we are testing turns out to be safe and effective, we may in the future very well be able to perform this kind of study on people who have diabetes-related autoantibodies but who have not yet developed the disease,” says Lundgren.
Ethical dilemma
Scientists still do not know what causes type 1 diabetes. Studies have shown a link between enterovirus infection and the development of autoantibodies and type 1 diabetes. Enterovirus is the collective name of viruses that reproduce in the throat and intestines before spreading to other organs. Research is underway to establish whether it is possible to develop a vaccine against type 1 diabetes that targets one of the viruses in that group.
“We hope to be involved if the research gets to the stage where the vaccine can be tested on children to prevent type 1 diabetes,” says Lernmark.
However, this type of research also raises ethical questions. Should researchers treat children who, although they carry autoantibodies, may never develop the disease? Diabetes researcher Olle Korsgren is professor of cell transplantation at Uppsala University and is involved in the strategic research area EXODIAB. He gives a couple of examples of ethical dilemmas.
“One potential dilemma is that if we treat a healthy individual with autoantibodies, the child may develop a sense of being ill. Another dilemma that can arise is that healthy participants who may never actually develop the disease instead experience side effects from the treatment. It is important that studies are conducted in an ethically acceptable manner, and that participants are given the right information,” he says.
At the same time, professor Korsgren is very positive about the way type 1 diabetes research is moving forward. He notes that many pharmaceutical companies choose to invest in clinical studies in type 2 diabetes, which is the most common form of the disease.
Source: Read Full Article