Yulan Xiong, assistant professor of neuroscience at UConn Health, and her team have discovered that a regulator compound holds the potential to treat Parkinson’s disease.
Scientists have known that, in most familial cases, Parkinson’s disease is caused by a genetic mutation in a gene called LRRK2.
This gene has multiple functions in the brain and other parts of the body including regulating cell function and transmitting signals.
With Parkinson’s disease, mutation to LRRK2 does not cause the protein it codes for, daradarin, to become deformed. Instead, the body begins producing too much of the protein.
Until now, scientists did not know how to control this protein expression because they didn’t understand the mechanisms underlying it.
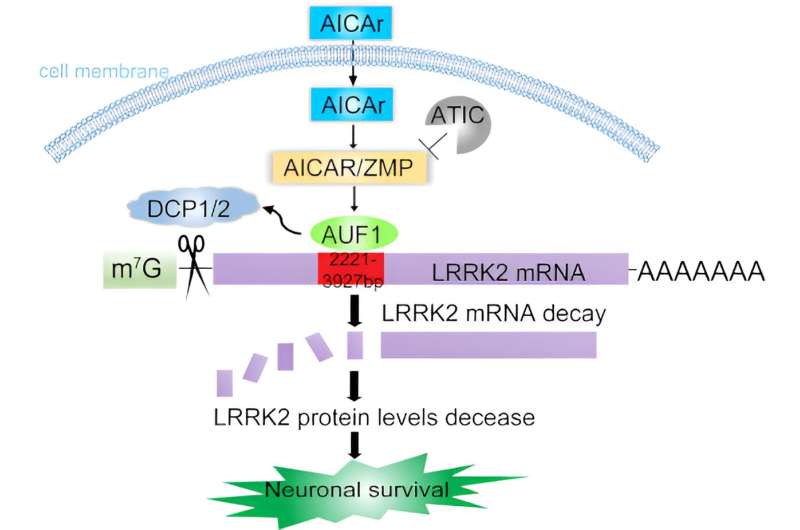
The Xiong lab has solved this mystery with their novel study identifying an LRRK2 regulator, an enzyme called ATIC, and a potential pharmaceutical treatment. Xiong recently published these findings in The EMBO Journal.
Xiong and her lab first performed a genome-wide screening to identify candidate genes that could be LRRK2 regulators in yeast cells.
Xiong and her Ph.D. student Qinfang Liu quickly realized that something important was going on at the mRNA level. When genes need to make a protein, they are copied into mRNA—the instructions to the rest of the cell for how to build the protein.
The ATIC enzyme was regulating LRRK2 at the mRNA level, not at the protein level.
“This was a surprising discovery,” Xiong says. “At the beginning we did a screening, we identified a candidate, and we found that it was targeted at the mRNA level. This is a new discovery for us too.”
The researchers then looked at ATIC in human neural cells, since Parkinson’s disease affects the brain, as well as fruit fly and mouse models.
ATIC is responsible for purine metabolism. Purines are nitrogen bases found in meat and seafood, as well as certain vegetables and grains.
ATIC substrate brings in a binding protein called AUF-1 to specific regions of LRRK2 mRNA. AUF-1 then recruits another DCP1/2 enzyme complex. Together they are able to reduce LRRK2 levels.
Xiong and her lab discovered that AICAr, the precursor of ATIC substrate, a drug that mimics ATIC activity, can significantly repress LRRK2 levels.
“We used a primary neuronal culture to see how those candidates can regulate LRRK2,” Xiong says. “And we found that it can significantly regulate the LRRK2 expression.”
Previous studies have focused on LRRK2’s enzymatic activity. Until now, no one had looked at its larger expression network.
“Our study is the first to find out the mechanism,” Xiong says. “It’s also important that we identified the compound, that can directly decrease LRRK2 levels which means that we can use this compound to treat Parkinson’s patients.”
AICAr has shown promise in preclinical trials as a treatment for metabolic disorders, cardiovascular diseases, and other conditions. But AICAr could not pass through the blood-brain barrier, a major limitation for its use treating Parkinson’s disease.
Xiong and her collaborators are currently working on modifying AICAr to overcome this challenge.
“We wanted to modify those structures that can bring this compound past the blood-brain barrier,” Xiong says.
Xiong and her lab are working with UConn’s Technology Commercialization Services (TCS) to protect and leverage this groundbreaking discovery with the goal to further advance and refine this technology for societal benefit. With TCS having already filed a non-provisional patent application for the technology, they are now facilitating connections between Xiong and prominent companies specializing in Parkinson’s disease treatment.
Xiong and her lab plan to continue animal model trials and hopefully move to clinical human trials in the near future.
More information:
Qinfang Liu et al, Regulation of LRRK2 mRNA stability by ATIC and its substrate AICAR through ARE‐mediated mRNA decay in Parkinson’s disease, The EMBO Journal (2023). DOI: 10.15252/embj.2022113410
Journal information:
EMBO Journal
Source: Read Full Article