A new study led by researchers at The University of Texas MD Anderson Cancer Center provides a deeper understanding of the evolution of the tumor microenvironment during gastric cancer progression. Highlights of the study, published today in Cancer Cell, include a link between multicellular communities and clinical outcomes as well as a potential new therapeutic target.
Gastric adenocarcinoma is one of the most lethal cancers worldwide due to inherent treatment resistance, but the cellular and molecular mechanisms involved in the progression from early pre-cancer to tumor formation and metastasis are not well understood. This research sheds light on how the various immune and stromal cell subsets evolve during gastric cancer development.
The study was conducted by Linghua Wang, M.D., Ph.D., associate professor of Genomic Medicine, in collaboration with Jaffer Ajani, M.D., professor of Gastrointestinal Medical Oncology, and Ruiping Wang, Ph.D., postdoctoral fellow in the Wang Lab.
“Gastric adenocarcinoma exhibits a high degree of heterogeneity with respect to both its phenotypes and molecular characteristics, but research around it has lagged behind other cancer types,” Wang said.
“Most studies have concentrated on tumor cells and largely overlooked the immune and stromal cells within the tumor microenvironment, which are very dynamic and play critical roles in cancer progression. This study represents the largest single-cell RNA sequencing cohort of gastric adenocarcinoma to date and brings important new insights into how these cell populations impact disease progression.”
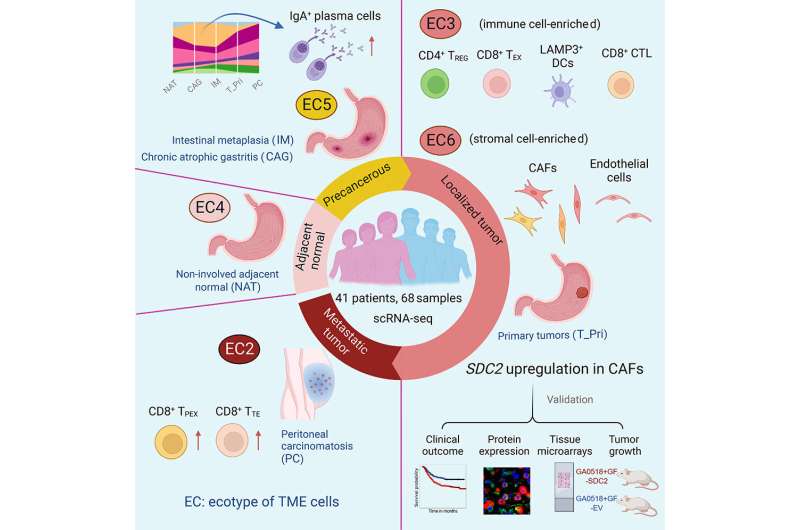
By obtaining single-cell RNA sequencing (scRNA-seq) data from 68 gastric adenocarcinoma samples encompassing various disease stages—including precancerous lesions, localized tumors and distant metastases—along with normal tissue and peripheral blood samples, the team characterized the diverse immune and stromal cell populations within the tumor microenvironment and discovered exploitable targets to modulate the tumor microenvironment.
Novel approach allows researchers to dissect the complex tumor microenvironment
Various immune and stromal cell subsets formed multicellular communities, or collections of cell states, present in the tumor microenvironment of an individual tumor sample. The research team termed these groups “ecotypes” and identified six unique ecotypes, with each dominated by specific immune and stromal cell states.
“While many published single-cell studies have focused on characterizing the heterogeneity of each individual cell compartment, our study utilized a novel approach and concept of integrating various components of the tumor microenvironment to define ecotypes and investigated their clinical significance,” Wang said. “This approach can readily be applied to studies in other cancer types.”
A notable discovery is that two ecotypes (EC3 and EC6) correlated with different histological, genomic and clinical features of primary gastric adenocarcinomas. Tumors categorized as EC3 were composed mainly of immune cell subsets, whereas EC6 tumors predominantly included stromal cell subsets. Patients with EC6 tumors had more aggressive disease and significantly shorter survival compared to those with EC3 tumors.
Findings point to SDC2 as potential therapeutic target in stromal cells
While stromal components within the tumor microenvironment play crucial roles in tumor initiation, progression and metastases, cancer treatment strategies have thus far rarely focused on modulating stromal components, especially in patients with gastric adenocarcinoma.
This study identified SDC2 as a potential target worthy of further investigation. Researchers found SDC2 overexpression in stromal cells, especially in cancer-associated fibroblasts, was correlated with aggressive disease and advanced stages, and strongly associated with unfavorable survival outcomes. In addition, SDC2 expression was consistently elevated in stromal cells across various other cancer types, including pancreatic cancer, colorectal cancer, bladder cancer, breast cancer and clear cell renal cell carcinoma.
“There are unmet needs for patients with gastric adenocarcinoma every step of the way in their clinical journey,” Ajani said. “Our team strives to use novel interrogations to discover new therapeutic targets to improve the outcomes of these patients. While there are many questions left to answer, targeting SDC2 in cancer-associated fibroblasts represents a potentially exciting avenue that warrants further investigation.”
The research team has shared their results with the wider research community through the online Single-Cell Research Portal developed by the Wang Lab.
More information:
Linghua Wang, Evolution of Immune and Stromal Cell States and Ecotypes during Gastric Adenocarcinoma Progression, Cancer Cell (2023). DOI: 10.1016/j.ccell.2023.06.005. www.cell.com/cancer-cell/fullt … 1535-6108(23)00215-5
Journal information:
Cancer Cell
Source: Read Full Article