Breast cancer spreading to other organs usually heralds a poorer prognosis. Researchers at the University and University Hospital of Basel have discovered a process that helps breast cancer cells implant themselves in certain places in the body. The results suggest a way of preventing secondary tumors.
For eight years, a team led by Professor Mohamed Bentires-Alj worked to establish the role of a cellular enzyme in breast cancer metastasis. The three lead authors Joana Pinto Couto, Milica Vulin, Charly Jehanno and collaborators discovered a mechanism that appears to support metastasis in a range of aggressive cancers. The team has reported their findings in The EMBO Journal .
A cell can be pictured like a social network: in theory, every person is connected to every other person in the world through surprisingly few degrees of separation. Cell factors in molecular networks are connected to each other in an analogous way. If one stops functioning correctly, the system is thrown out of balance.
The result is a cascade of effects that can have wide-ranging and unexpected consequences on more distant parts of the network. Deciphering these cascades can contribute to our understanding of how a minordefect in a cell’s system can lead to diseases like cancer. These insights offer ideas for new treatments.
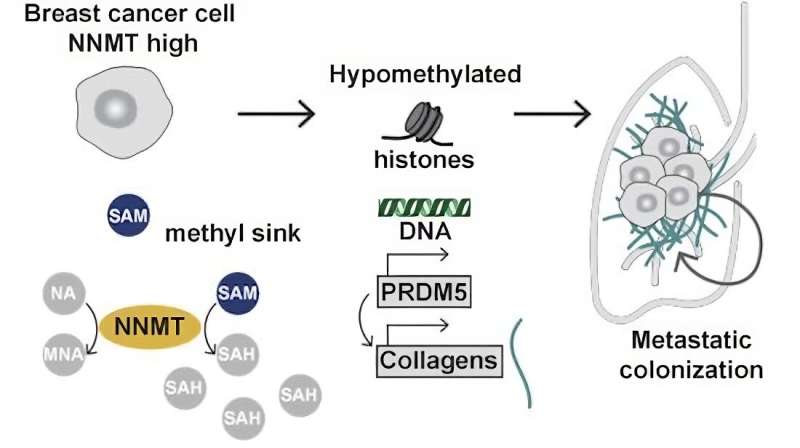
Bentires-Alj’s research team at the Department of Biomedicine, University of Basel and University Hospital Basel, elucidated one of these cascades. It begins with a metabolic enzyme called nicotinamide N-methyltransferase, or NNMT for short. And it ends with the substance that fills the space between the body’s cells and holds them together: collagen. Collagen is actually a good thing. But in the case of metastatic cancer, it betrays the body and helps cancer cells embed themselves in new tissues.
Wandering cancer cells with their own collagen
“Triple negative” breast cancer, which affects roughly 15 percent of all breast cancer patients, is particularly aggressive because it often spreads throughout the body and forms lung and brain metastases. These breast cancer cells produce unusually high amounts of NNMT. As the researchers learned through experiments on animals, overproduction of NNMT is key to the metastasis.
Why? The answer is found at the end of the cascade, with collagen. As the Basel research team reports, overproduction of NNMT causes the cancer cells to also produce more collagen than normal.
It is known from previous studies that wandering cancer cells first have to find their way around in new tissues. The environment there—that is, the semiochemicals and available nutrients and oxygen—is different from that of the original tumor. In this preliminary stage of metastasis, the collagen in the new tissue helps the cancer cells survive and adapt.
What the new study found is particularly aggressively metastasizing breast cancer cells not only produce an excessive amount of NNMT, but also their own collagen. “This ability makes them less dependent on the collagen of the new tissue, so it’s even easier for the cancer cells to entrench themselves,” explains Dr. Charly Jehanno, one of the study’s first authors.
No NNMT, no collagen
When the researchers removed NNMT from aggressive breast cancer cells and injected these cells into mice, the animals developed hardly any metastases. The cells also produced hardly any collagen.
A literature review also found that overproduction of NNMT is characteristic of a whole range of aggressive cancers—meaning it could be a universally important key factor in cancer metastasis.
“Next, we want to test whether existing NNMT inhibitors can also stop metastases in mouse models and whether they have any side effects,” explains Mohamed Bentires-Alj. Following further development of NNMT targeting agents, the first studies in human patients could follow.
More information:
Joana Pinto Couto et al, Nicotinamide N‐methyltransferase sustains a core epigenetic program that promotes metastatic colonization in breast cancer, The EMBO Journal (2023). DOI: 10.15252/embj.2022112559
Journal information:
EMBO Journal
Source: Read Full Article