Injuries to the nerves can blind or paralyze because adult nerve cells don’t regenerate their connections. Now, a team of UConn School of Medicine researchers report in Development that in everyone there exists at least a small population of nerve cells that could be coaxed to regrow, potentially restoring sight and movement.
Glaucoma; optic neuritis; trauma or stroke of the optic nerve: All of these conditions can irreversibly damage the optic nerve, leading to blindness. Glaucoma alone affects more that 3 million people in the U.S. Nerve damage leading to paralysis is similarly common, with around 5 million people in the US living with some form of it, according to the Christopher Reeve Foundation.
Although blindness and paralysis may seem quite different, many types of these two conditions share the same underlying cause: nerves whose axons, the long fibers that connect the nerve to the brain or spinal cord, are severed and never grow back. Axons act like wires, conducting electrical impulses from various parts of the body to the central nervous system. If a wire is cut, it cannot transmit signals and the connection goes dead. Similarly, if the axons in the optic nerve cannot reach the brain, or the axons from your toe cannot connect to the spinal cord, you will not be able to see from that eye or move your toe.
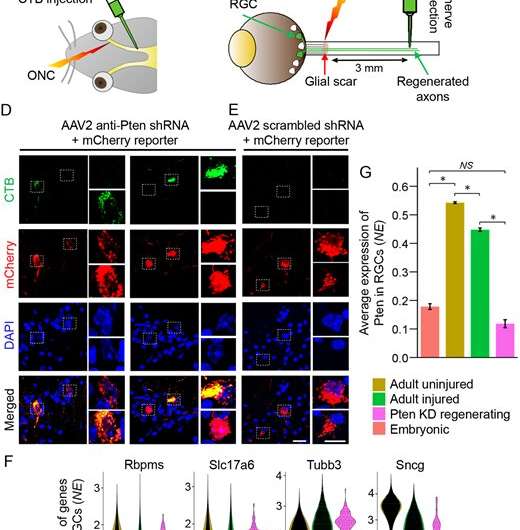
Some animals can regrow axons, but mammals such as mice and humans cannot. It was assumed that mammals lack the immature nerve cells that would be needed. But a team of researchers in UConn School of Medicine neuroscientist Ephraim Trakhtenberg’s lab has found otherwise. In an April 24 paper in Development, they report the existence of neurons that behave similarly to embryonic nerve cells. The neurons express a similar subset of genes, and can be experimentally stimulated to regrow long-distance axons that—under the right circumstances—could lead to healing some vision problems caused by nerve damage.
Moreover, the researchers found that mitochondria-associated Dynlt1a and Lars2 genes were upregulated in these neurons during experimental axon regeneration, and that activating them through gene therapy in injured neurons promoted axon regeneration, thereby identifying these genes as novel therapeutic targets. Trakhtenberg believes that similar immature nerve cells exist in regions of the brain outside the visual system too, and might also heal some features of paralysis under the right circumstances.
The right circumstances are difficult to provide, though. Once stimulated by a treatment, these embryonic-like nerve cells’ axons start to regrow in injured areas, but tend to stall before they reach their original targets. Previous research has shown a combination of cell maturity, gene activity, signaling molecules within the axons, as well as scarring and inflammation in the injury site, all seem to inhibit axons from regrowing. Some therapies that target genes, signaling molecules, and injury site environment can encourage the axons to grow somewhat, but they rarely grow long enough.
Researchers in the Trakhtenberg lab began looking at how another type of cell, oligodendrocytes, were behaving. If axons are the wires of the nervous system, oligodendrocytes make the insulation. Called myelin, it insulates the axons and improves conductivity. It also—and this is key—prevents the axons from growing extra, extraneous connections. Typically, axons in embryos grow to their full length before they are coated with myelin. But postdoctoral fellow Agniewszka Lukomska, MD/Ph.D. student Bruce Rheaume, graduate student Jian Xing, and Trakhtenberg found that in these injury sites, the cells that apply myelin start interacting with the regenerating axons shortly after they begin growing. That interaction, which precedes the insulation process, contributes to the axons stalling out, so that they never reach their targets.
The researchers describe this finding in an April 27 paper in Development. The researchers suggest that a multi-pronged approach would be needed to fully regenerate injured axons. Therapies that target both the gene and signaling activity within the nerve cells would be necessary to encourage them to grow as an embryonic nerve cell would. Clearing the environment of inhibitory molecules and pausing oligodendrocytes from insulating would give the axons time to reconnect with their targets in the central nervous system before being myelinated. Then, treatments that encourage oligodendrocytes to myelinate the axons would complete the healing process.
Although in some types of complex injures protection by myelination of still intact but demyelinated axons from ensuing inflammatory damage may take precedence, ultimately secondary inflammatory damage may be controlled pharmacologically, paving the way for pausing myelination and unhindering therapeutic axon regeneration for these types of lesions as well, Trakhtenberg says.
The new insights into how axons grow could someday create a path for truly effective therapies for blindness, paralysis and other disorders caused by nerve damage. But for Trakhtenberg, the research has even deeper significance. It answers some of the big questions of how our nervous systems develop.
“If you succeed in regenerating injured neural circuits and restoring function, this would indicate that you are on the right track toward understanding how at least some parts of the brain work,” Trakhtenberg says. The researchers are currently working on a deeper understanding of the molecular mechanisms behind both axon growth and interaction with oligodendrocytes.
More information:
Bruce A. Rheaume et al, Pten inhibition dedifferentiates long-distance axon-regenerating intrinsically photosensitive retinal ganglion cells and upregulates mitochondria-associated Dynlt1a and Lars2, Development (2023). DOI: 10.1242/dev.201644
Jian Xing et al, Post-injury born oligodendrocytes incorporate into the glial scar and contribute to the inhibition of axon regeneration, Development (2023). DOI: 10.1242/dev.201311
Journal information:
Development
Source: Read Full Article