Despite racial and ethnic minority groups making up nearly half of the United States population, underrepresentation in clinical trials remains a critical challenge. In an effort to improve clinical trial diversity, researchers at The Ohio State University Wexner Medical Center and College of Medicine partnered with The African American Male Wellness Agency, Genentech Inc. and Pfizer, Inc. to engage with almost 450 community members in 25 states and five countries to create solutions to barriers of access, awareness, discrimination and racism and workforce diversity.
Study findings are published online in the journal PLOS ONE.
“Equitable representation is key when testing novel therapeutic and non-therapeutic interventions to ensure safety and effectiveness across populations, especially since 20% of new drugs demonstrate differences in exposure and/or response across racial and ethnic groups,” said senior author Dr. Joshua Joseph, assistant professor of medicine in the Division of Endocrinology, Diabetes and Metabolism and an investigator in the Diabetes & Metabolism Research Center.
“The lack of Black and Hispanic/Latinx populations in clinical research studies for endocrine conditions including diabetes, cancer, and cardiovascular disease research is particularly troubling because these diseases are common with a high prevalence and mortality in racial and ethnic minority populations,” said co-author Timiya Nolan, assistant professor of nursing and principal investigator of Partners in Negating Statistics in Black Women (PINS).
During the study in 2021, participants attended two webinars in a four-part series titled “Health Equity Through Diversity: From Communities to Clinics to Clinical Trials.” They discussed solutions for advancing health equity through diversifying clinical trials and addressing medical mistrust in communities.
Each 90-minute webinar began with panelist discussions followed by breakout rooms where moderators led discussions related to health equity while scribes recorded the conversations. The diverse groups of panelists included community members, civic representatives, clinician-scientists, government organizations and biotechnology/biopharmaceutical professionals. Scribe notes from discussions were collected and thematically analyzed to uncover the central themes.
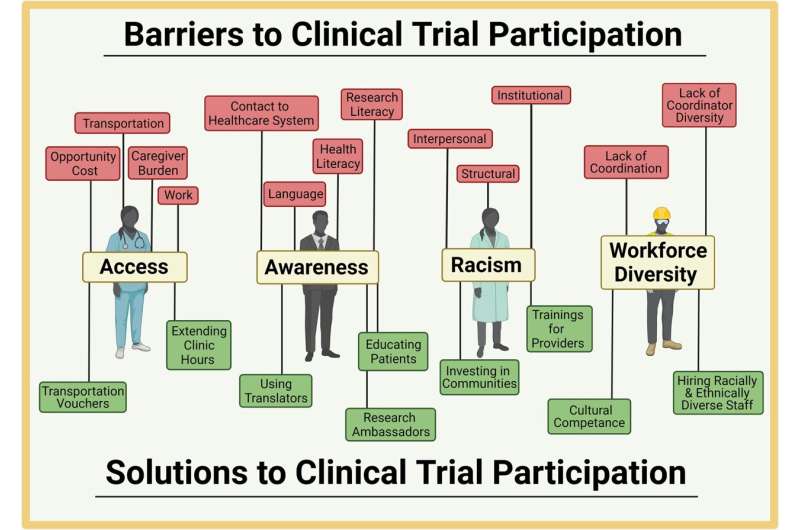
“We found that based on these discussions, barriers to clinical trial participation were broadly grouped into the themes of access, awareness, discrimination and racism and workforce diversity. Participants noted that innovative, community-engaged, co-designed solutions are essential,” said first author Luiza Reopell, clinical study coordinator with the Division of Endocrinology, Diabetes and Metabolism.
Within each theme, barriers and solutions to clinical trial participation were identified:
- Access: Barriers included opportunity cost, transportation, caregiver burden and work. Solutions included transportation vouchers and extending clinic hours.
- Awareness: Barriers included contact with healthcare system, research literacy, language and health literacy. Solutions included using translators or research ambassadors and educating patients.
- Racism: Barriers included interpersonal, structural and institutional racism. Solutions included investing in communities and offering training for providers.
- Workforce Diversity: Barriers included lack of coordination and lack of clinical research coordinator diversity. Solutions included hiring racially and ethnically diverse staff and ensuring cultural competence.
More information:
Luiza Reopell et al, Community engagement and clinical trial diversity: Navigating barriers and co-designing solutions—A report from the “Health Equity through Diversity” seminar series, PLOS ONE (2023). DOI: 10.1371/journal.pone.0281940
Journal information:
PLoS ONE
Source: Read Full Article