In a recent study posted to the medRxiv* preprint server, researchers evaluated the impact of changes in infection fatality rate (IFR), intrinsic reproductive number (R0), and the durability of immunity on the annual coronavirus disease 2019 (COVID-19) death toll in the United States (US). This study demonstrated the risks of abiding by the ‘live with the disease’ COVID-19 mitigation strategy.
In the present-day scenario, solely relying on vaccines to put the COVID-19 pandemic to an end seems unfeasible due to waning immunity and the emergence of several immune-evasive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants. The ‘live with the disease’ strategy is now being employed by health administrations and involves reducing COVID-19 severity while permitting uninhibited SARS-CoV-2 transmission, with the expectation that the future SARS-CoV-2 variants would have milder outcomes due to viral attenuation or progressive build-up of immunity.
 Study: Endemicity is not a victory: the unmitigated downside risks of widespread SARS-CoV-2 transmission. Image Credit: FrankHH / Shutterstock
Study: Endemicity is not a victory: the unmitigated downside risks of widespread SARS-CoV-2 transmission. Image Credit: FrankHH / Shutterstock
About the study
The present work explores the strategic implications of permitting widespread viral transmission while relying on vaccines to limit short-term morbidity and mortality. The researchers assessed the impact of changes in SARS-CoV-2 virulence on its transmissibility. They also evaluated the effects of IFR alterations of the endemic SARS-CoV-2 variants on the annual COVID-19 mortality rates in the US.
For calculating the viral fitness disadvantage incurred by COVID-19 fatalities, the team estimated the fractional loss of transmission that occurred on the death of a SARS-CoV-2-positive patient.
To determine the loss of transmission that occurred on the death of a COVID-19 patient, the team converted the probability distribution function (PDF) of time from symptom onset to death to a cumulative distribution function (CDF) representing the likelihood of survival over time.
Subsequently, they calculated the product of viral transmissibility and survival distributions to determine the probability of transmission before death, given that both events occur. The loss of transmission due to a fatal outcome was calculated as 1 – the fractional transmissibility before the fatal outcome. The endemic COVID-19 condition was defined as that occurring when steady-state levels of disease spread are reached and maintained since the level of population immunity is equal to the herd immunity threshold (R0 – 1)/ R0.
The loss of transmissibility occurred in the fraction of infections that resulted in fatal outcomes, estimated as the IFR. Thus, the overall loss of transmissibility was the IFR multiplied by the fractional loss of transmissibility in fatal cases.
For determining the impact of changes in SARS-CoV-2 properties such as the R0, IFR, and duration of natural immunity on the annual US death tolls and infection rates, the team varied these parameters in a susceptible-exposed-infectious-recovered-susceptible (SEIRS) model, under several vaccination scenarios: 90%, 50%, or 0% decrease in risk of infection (VEi) with 100% or 70% vaccine uptake. They assumed that vaccination reduces the risk of death given infection (VEm) and the risk of infection (VEi) but has no additional benefit in preventing transmission in breakthrough cases.
Results
The expected loss of infectivity incurred on the death of a patient was approximately 1.3% of that individual’s overall propensity to transmit. Therefore, the overall loss of transmissibility for a lethal strain of SARS-CoV-2 was 1.3* IFR, assuming that the PDFs for infectivity and the fatal outcome would remain unaltered.
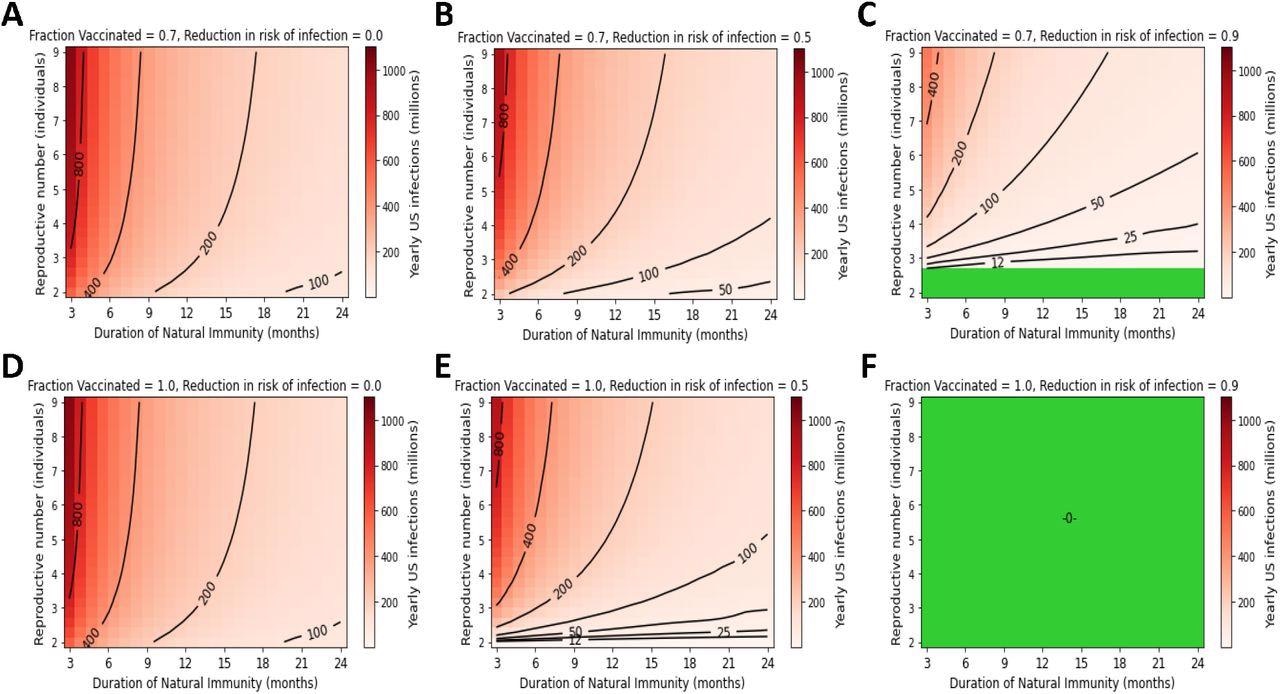
High yearly US infection counts persist under endemic conditions without vaccines that prevent transmission. Yearly US SARS-CoV-2 infections under the following conditions for vaccine compliance and VEi: A) 70% vaccinated with 0% VEi, B) 70% vaccinated with 50% VEi, C) 70% vaccinated with a 90% VEi, D) 100% compliance with a vaccine with 0% VEi, E) 100% compliance with 50% VEi, F) 100% compliance with 90% VEi. Green regions represent complete suppression of SARS-CoV-2 transmission.
Changes in IFR did not substantially affect the viral transmissibility. This indicates that there was no evolutionary pressure that favored a decrease in COVID-19 severity. Furthermore, the trajectory of SARS-CoV-2 evolution revealed that a higher IFR was not associated with a faster timeline to death or decreased transmission. As an example, the Delta variant with 80% higher transmissibility also had a 50% greater risk of death.
Considering 70% vaccination that decreased the COVID-19 risk by 90%, an R0 of 5, and an 18-month duration of natural immunity would result in over 50 million yearly infections, whereas an R0 of 8 and a 9-month duration of natural immunity would result in about 100 million infections.
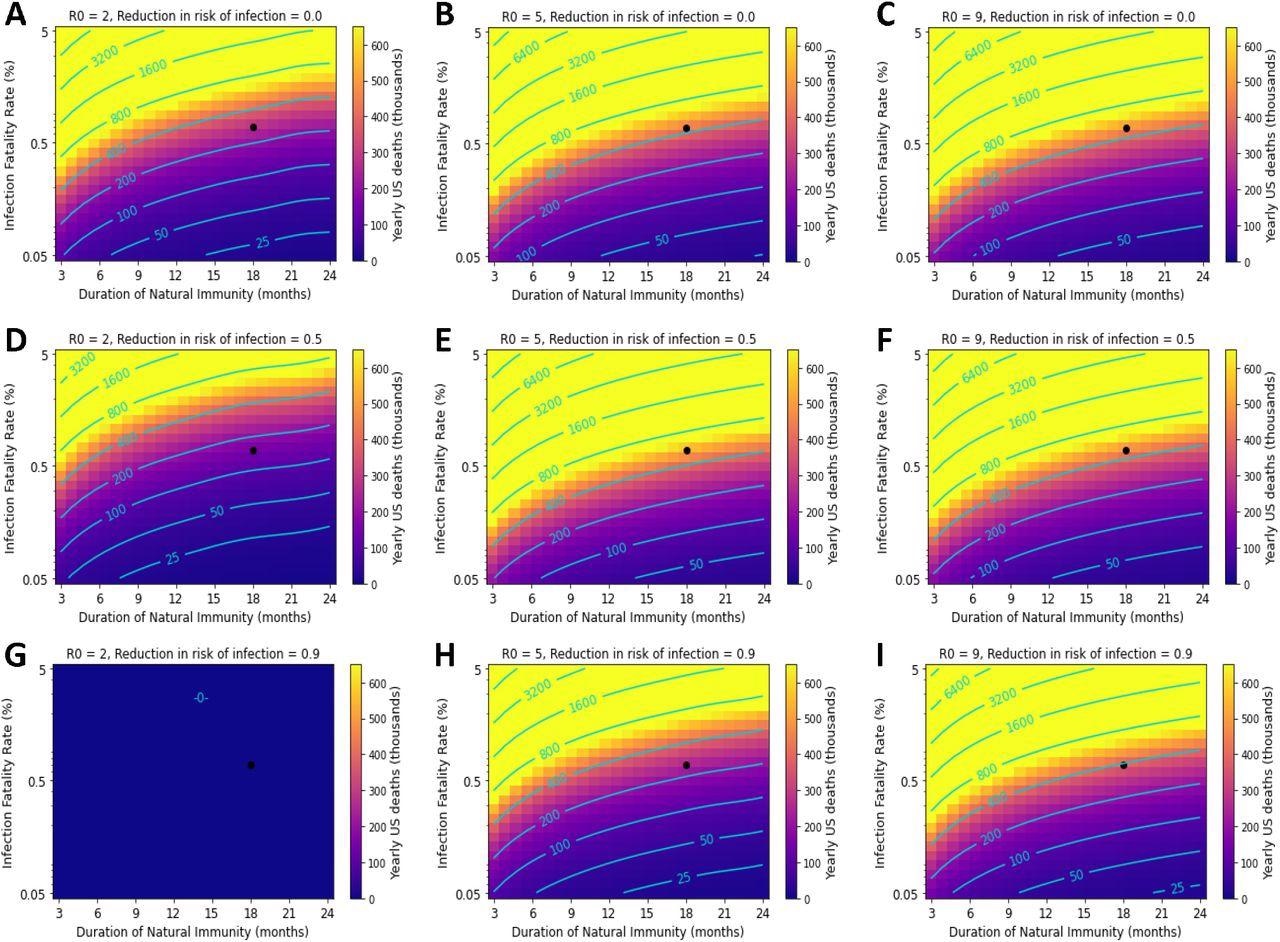
Variation in the duration of natural immunity and IFR can result in catastrophic death tolls. The black point represents parameter values corresponding to best-estimates of immunity and IFR for ancestral SARS-CoV-2. Yearly US COVID-19 deaths under the following transmissibility (R0) and VEi conditions: A-C) 0% VEi and R0 of 2, 5, and 9; D-F) 50% VEi and R0 of 2, 5, and 9. G-I) 90% VEi and R0 of 2, 5, and 9. Vaccine compliance is 70% and VEm is 90% in all panels.
The team observed that minor losses in vaccine effectiveness could result in a substantial increase in population-level COVID-19 mortality. For example, the model predicted that the COVID-19 mortality under best-estimate parameters (R0 of 5, 70% vaccine coverage, and 18-month natural immunity with a 50% Vei vaccine) was nearly 400,000 if the vaccine prevented 90% of COVID-19 deaths. If the vaccine’s VEm is reduced to 70%, there would be a 50% increase in COVID-19 deaths, approaching 600,000 per year.
For a SARS-CoV-2 variant with R0 of 5, 1% IFR, and a 12-month duration of natural immunity, about 700,000 deaths were predicted annually if a vaccine prevented 90% of infections were administered to 70% of the population. This indicates that even a slight change of 1% in IFR could significantly impact the annual COVID-19 death toll.
Overall, the study findings showed that increased viral virulence would cause minimal loss of transmission. In addition, SARS-CoV-2 implies extensive transmission, which results in hundreds of thousands of deaths annually in the US under several plausible scenarios, with even a modest increase in IFR giving rise to an unsustainable mortality burden.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Endemicity is not a victory: the unmitigated downside risks of widespread SARS-CoV-2 transmission. Madison Stoddard, Alexander Novokhodko, Sharanya Sarkar, Debra Van Egeren, Laura F. White, Natasha S. Hochberg, Michael Rogers, Bruce Zetter, Diane Joseph McCarthy, Arijit Chakravarty, medRxiv preprint 2022, DOI: https://doi.org/10.1101/2022.03.29.22273146, https://www.medrxiv.org/content/10.1101/2022.03.29.22273146v1
Posted in: Medical Science News | Medical Research News | Disease/Infection News
Tags: Coronavirus, Coronavirus Disease COVID-19, covid-19, Evolution, immunity, Mortality, Pandemic, Respiratory, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine

Written by
Pooja Toshniwal Paharia
Dr. based clinical-radiological diagnosis and management of oral lesions and conditions and associated maxillofacial disorders.
Source: Read Full Article