The Food and Drug Administration is getting closer to approving the country's first COVID-19 vaccine after its advisory group found that Pfizer’s vaccine is safe and effective in preventing the virus.
In documents released Tuesday morning, the FDA said the vaccine, which requires two shots administered three weeks apart, is more than 50 percent effective after just the first dose, and nearly 100 percent effective after the second. And that effectiveness is true across all age groups and ethnicities, and for people with preexisting conditions such as obesity, high blood pressure and diabetes.
The promising results will likely lead the FDA to approve Pfizer’s vaccine on Thursday, when the Vaccines and Related Biological Products Advisory Committee meets to vote on whether to approve it for emergency use.
Pfizer’s vaccine is already in use in the United Kingdom, where the first recipients of the vaccine were inoculated Tuesday morning. A 90-year-old grandmother, Margaret Keenan, received the first dose in England, and said she feels “so privileged to be the first person vaccinated against COVID-19,” the Associated Press reported. "It's the best early birthday present I could wish for because it means I can finally look forward to spending time with my family and friends in the New Year after being on my own for most of the year."
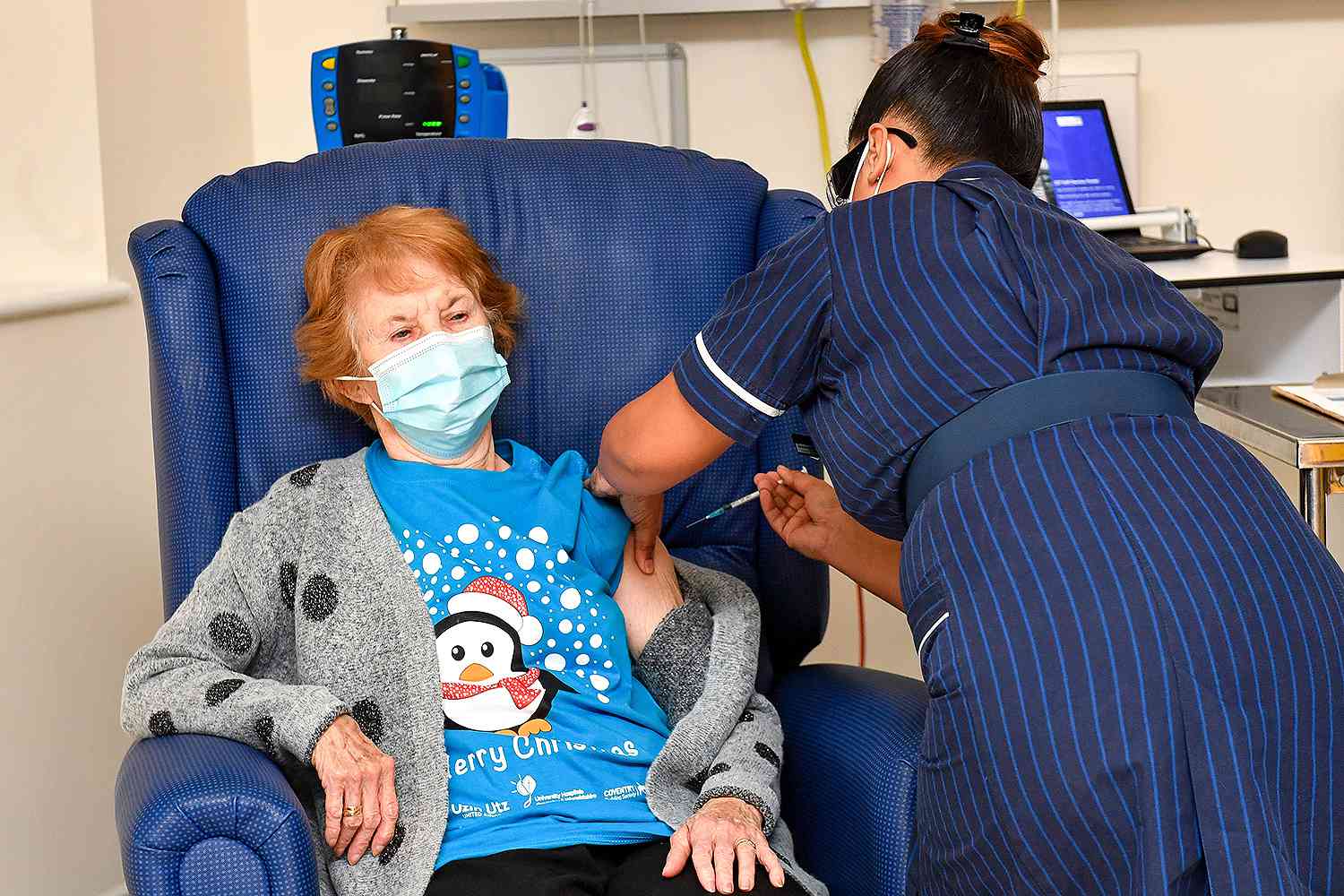
The second dose went to a man named William Shakespeare.
The U.S. has purchased 100 doses of Pfizer’s vaccine so far, enough to vaccinate 50 million people because of the two-dose regimen. The Trump administration reportedly passed on buying more doses of the vaccine over the summer, according to reports from The New York Times and the Washington Post, leading to concerns that there will not be enough doses to fulfill their plans to vaccinate most Americans by May or June.
According to the Post, Pfizer had recommended that the White House’s Operation Warp Speed vaccine program purchase 200 million doses, which would be enough for 100 million Americans. But officials declined, opting instead to buy 100 million doses, and the Times said that the White House is now trying to reverse course and buy up more doses that Pfizer may not have. Pfizer has since sold a significant amount of their vaccine supply to other countries.
The pharmaceutical company said that they may be able to give the U.S. another 50 million doses around the end of June 2021, and 50 million more by about the end of September.
In a press briefing on Monday, senior Trump administration officials denied that the White House turned down offers from Pfizer to buy more vaccine doses. They said that with several other vaccines other than Pfizer’s in development — including Moderna’s which is also on track for FDA emergency use approval —they are “absolutely confident we’ll have enough doses to vaccinate the American people by the end of the second quarter, 2021.”
The White House has purchased 100 million doses from Moderna, enough for 50 million people, but administration officials said that they are currently in negotiations to purchase more vaccine doses from other manufacturers and declined to speak further.
As information about the coronavirus pandemic rapidly changes, PEOPLE is committed to providing the most recent data in our coverage. Some of the information in this story may have changed after publication. For the latest on COVID-19, readers are encouraged to use online resources from CDC, WHO, and local public health departments. PEOPLE has partnered with GoFundMe to raise money for the COVID-19 Relief Fund, a GoFundMe.org fundraiser to support everything from frontline responders to families in need, as well as organizations helping communities. For more information or to donate, click here.
Source: Read Full Article