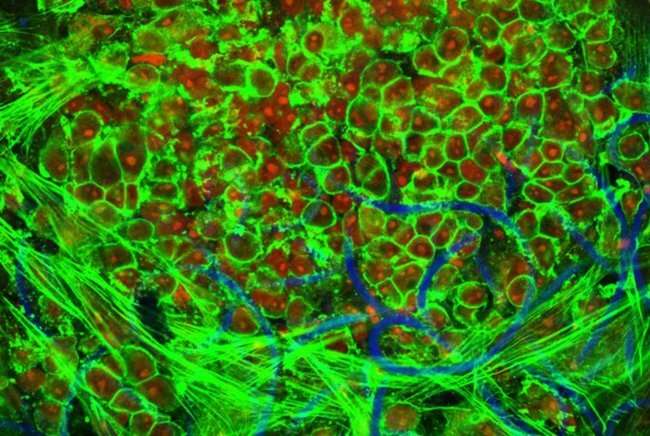
Chronic kidney disease affects the lives of millions of people. It’s a condition marked by the loss of several key kidney functions such as removing toxins from the blood. To compensate for this loss, a patient can undergo regular dialysis to clean their blood, which, in some cases, requires the implantation of a synthetic tube to connect to the dialysis machine. However, this tube can be blocked or obstructed by irregular cell growth, which in turn can negatively affect dialysis. Researchers at Eindhoven University of Technology in collaboration with Maastricht University have precisely studied how new tissue grows near these tubes. The paper is published in Nature Communications Biology.
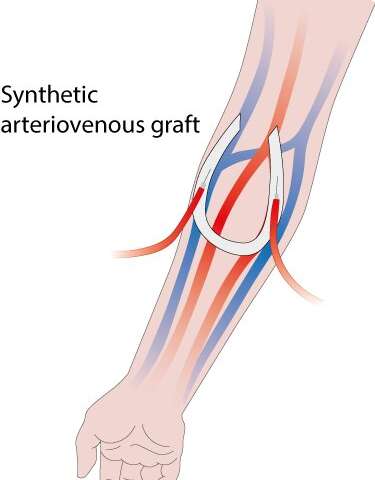
Patients with end-stage chronic kidney disease (or renal disease) must undergo frequent hemodialysis to remove toxins from their blood. During the treatment, the patient’s blood flows through a dialysis machine, which acts as an artificial kidney outside the body. This treatment needs a stable and reliable access point to the patient’s blood circulation system, also known as ‘vascular access,” which is added to the body via surgery
In the procedure, an artery (which carries oxygen-rich blood) and a vein (which carries blood low in oxygen) in the arm are joined together by connecting them directly to each other or via a synthetic tube known as an arteriovenous (AV) graft.
“In the case of AV grafts, they do their job, but unfortunately, they can present issues,” says lead author Eline van Haaften who worked with Carlijn Bouten and Nicholas Kurniawan from TU/e on this research. “Near the vein they can become blocked due to the growth of excessive amounts of vascular tissue. The driving factor for this process is thought to be the abnormal flow behavior of blood near the vein.”
Specialization
Designing synthetic grafts is a specialization of the group of Carlijn Bouten, professor in the Department of Biomedical Engineering at TU/e, and there is something rather special about these grafts.
They are based on scaffolds or temporary structures that can be placed inside the body. These structures encourage cells to grow on it. And the scaffolds are bioresorbable, meaning that they are absorbed by living tissue over time. “To prevent these self-regenerating vascular access grafts from becoming blocked inside the body, we need to better understand how blood flows near healthy and excessive vascular tissue,” says Bouten.
Taking a numerical-experimental approach
To explore this issue, the TU/e researchers turned to a numerical-experimental approach. They teamed up with the group of Wouter Huberts from the department of Biomedical Engineering at Maastricht University, who specializes in patient-specific mathematical models for clinical decision making.
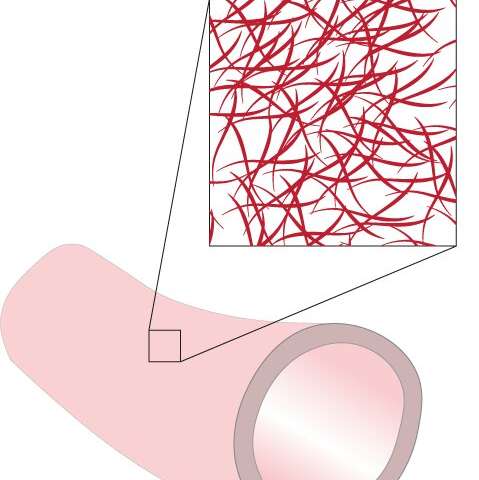
First, with patient data, they used numerical models designed by Sjeng Quicken to analyze the biomechanics at the juncture of the vein and graft. Then the researchers used this biomechanical data to plan experiments with an in vitro laboratory setup that can mimic how vascular tissue grows near the graft.
To best approximate the environment inside the body, they cultured a self-regenerating vascular access graft in a bioreactor that allowed for precise control over the blood-flow induced shear stresses and strains experienced by the graft. In this case, shear stresses can deform the tissue in a sideways direction.
The researchers observed that pulsating or oscillatory shear stresses lead to blocking around the graft as well as excessive and chaotic tissue growth. Minimizing the shear stresses in vivo could help to significantly decrease the negative effects associated with excessive tissue growth. Importantly, these results can help in optimizing the design of future regenerative grafts.
Engineering strengths to the fore
This work is significant in that it combines computational modeling and experiments, and Carlijn Bouten is quick to point this out: “This research showcases our engineering strengths in regenerative medicine. We’ve combined experimental and computational approaches to better understand a key clinical issue in regenerative medicine.”
And co-author Nicholas Kurniawan is also enthused by the results from the study: “This is a demonstration of a bottom-up approach. We’ve used patient data to tune a computer model, and then used data from the model to dictate experiments. This is a powerful approach towards identifying the key biophysical and mechanobiological processes that we can’t normally monitor in vivo.”
Source: Read Full Article