Several studies have shown the effectiveness of the BioNTech/Pfizer messenger ribonucleic acid (mRNA) BNT162b2 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine. Two doses of the vaccine have been shown to provide a short-term effect.
In a new study published in Nature Communications, a group of scientists from Israel sought to evaluate the long-term effectiveness of the BioNTech/Pfizer mRNA BNT162b2 vaccine. To this end, they assessed the correlation between time-from-vaccination and incidence of breakthrough infection within the specified study period.
 Study: Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Image Credit: Marco Lazzarini / Shutterstock.com
Study: Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Image Credit: Marco Lazzarini / Shutterstock.com
Duration of protection by vaccination
The Delta variant of SARS-CoV-2 was first identified in India; however, it is now to dominant circulating strain globally. Recently, several vaccinated individuals have reported SARS-CoV-2 infection, which has increased concerns about reduced vaccine efficacy against the Delta variant. Some studies report moderate differences in vaccine effectiveness and considerable antibody response against the Delta variant.
Taken together, it is important to evaluate the duration of protection provided by vaccines. This will help in effective resource allocation and vaccine administration. Furthermore, this information will also help in deciding the need for a third dose for longer protection.
A retrospective study
Israel had a rapid rollout of its mass vaccination campaign. The Maccabi Healthcare Services (MHS), which is Israel’s second-largest Health Maintenance Organization, has a centralized computerized database that covers 2.5 million members, which is about 26.5% of the population and provides a representative sample of the Israeli population. This data provided a window of opportunity to investigate the correlation between time-from-vaccination and vaccine effectiveness against the Delta variant.
The study population included all MHS members aged 16 and above who received the second dose of the Pfizer-BioNTech vaccine between January and April 2021. Individuals were considered fully vaccinated if they received two doses of the BNT162b2, with the second one administered within the 21-to-28-day interval set by national guidelines.
Individuals were excluded from the study if they had a positive SARS-CoV-2 polymerase chain reaction (PCR) assay test result before the start of the study period or disengaged from MHS for some reason between January and April. Dates of the first and second dose of the vaccine and results of any PCR tests for SARS-CoV-2 were included in the analysis.
Statistical models were employed to assess the correlation between time-from-vaccination and protection against breakthrough infection. In the analysis, the outcome was defined as a positive SARS-CoV-2 PCR test recorded between June 1, 2021 and July 27, 2021, which was the date of analysis.
Depending on the time-from-vaccination, the individuals were grouped into two separate groups including Early Vaccinees and Late Vaccinees. Early Vaccinees were individuals who received the second dose of the vaccine between January and February 2021, whereas Late Vaccinees were individuals who received the second dose between March and April 2021.
Vaccine waning with time
MHS has 1,395,134 members over the age of 16 and 1,352,444 who were eligible for the study. The incidence rates of breakthrough infections per 10,000 individuals who received their second dose according to the months were as follows: January – 36.5, February – 33.65, March – 23.06, and April – 16.98.
Between June 1 and July 27, 2021, a total of 1,911 cases of breakthrough infection were recorded. Of them, 1,151 were in the Early Vaccinees group and 760 were in the Late Vaccinees group.
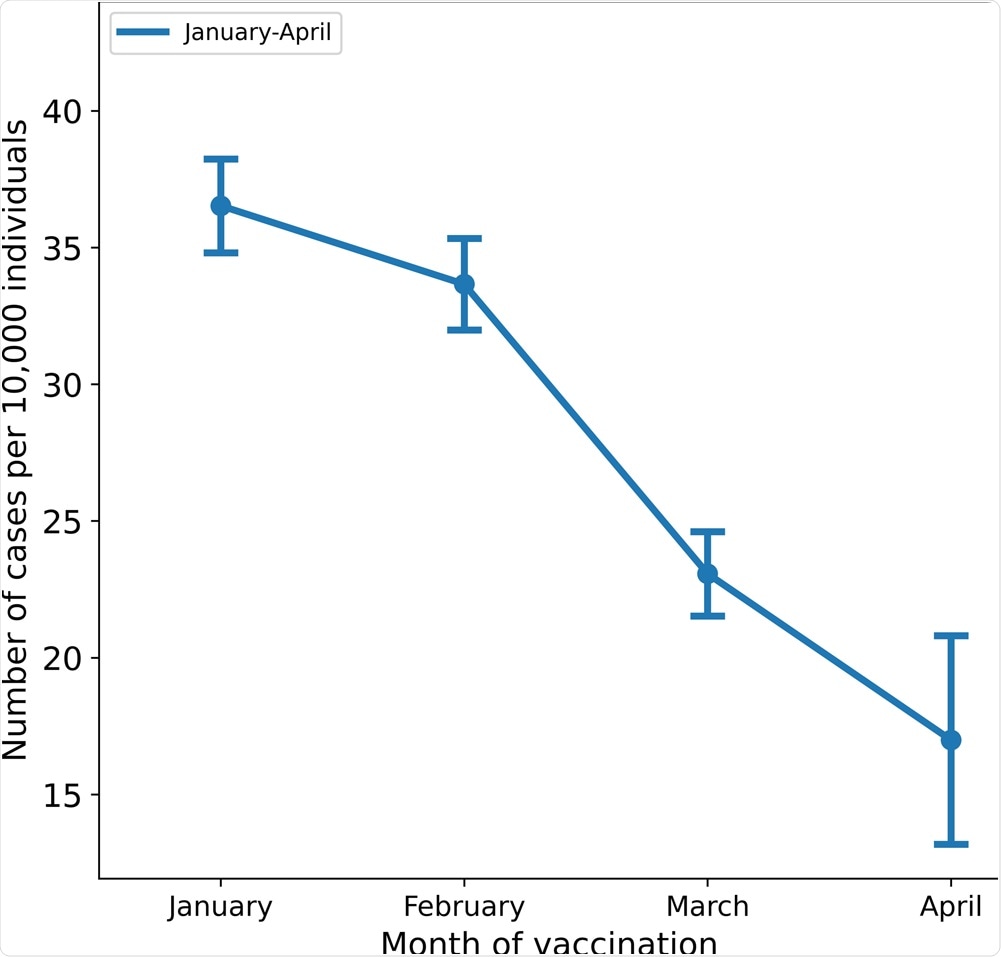 The crude incidence rates per 10,000 individuals above the age of 16 are shown by the month of administration of the second dose of the vaccine; bars represent 95% confidence intervals.
The crude incidence rates per 10,000 individuals above the age of 16 are shown by the month of administration of the second dose of the vaccine; bars represent 95% confidence intervals.
In the analysis, the scientists controlled for potential confounders like age and comorbidities. The analysis showed that the Early Vaccinees had a 1.51-fold increased risk of infection when compared to the Late Vaccinees.
The results were similar across all ages groups. This increased risk reached 2.26-fold when comparing the individuals vaccinated in January to those vaccinated in April.
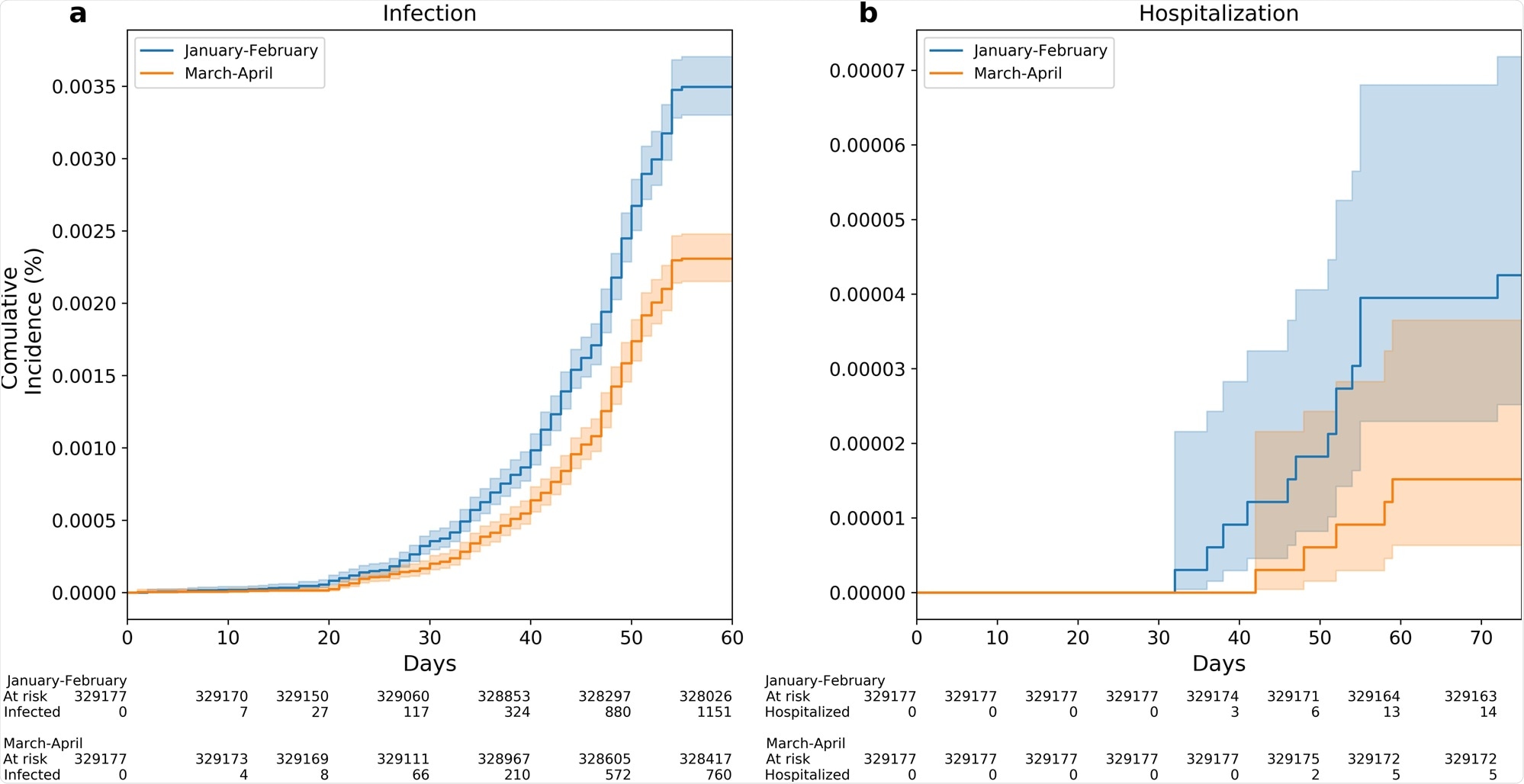 Kaplan–Meier curves are used for the accumulative probability of breakthrough infection (a) and hospitalization (b) between June and July 2021 among individuals who were vaccinated Early (January to February 2021) compared with individuals who were vaccinated late (March to April 2021). X axis in curves a, b represents days from the start of the follow-up period (June 1, 2021). Shading illustrates 95% CIs (a, b).
Kaplan–Meier curves are used for the accumulative probability of breakthrough infection (a) and hospitalization (b) between June and July 2021 among individuals who were vaccinated Early (January to February 2021) compared with individuals who were vaccinated late (March to April 2021). X axis in curves a, b represents days from the start of the follow-up period (June 1, 2021). Shading illustrates 95% CIs (a, b).
Conclusions
The current study showed a significant correlation between time-from-vaccination and protection against SARS-CoV-2 infection. The risk of a breakthrough infection was significantly higher for Early Vaccinees compared to the Late Vaccinees, with an additional trend of a higher risk for hospitalization among the Early Vaccinees group.
These results corroborate findings from recent studies that demonstrate a significant decline in antibody levels and immune levels over time following the second dose of vaccination.
This study presents a preliminary finding which should be evaluated further. Future studies should examine the long-term protection of current coronavirus disease 2019 (COVID-19) vaccines against different SARS-CoV-2 strains. Further, there should be prospective clinical trials to examine the effect of a booster dose against breakthrough infection.
Limitations
The inference drawn from this study may not correlate to the protection afforded by the vaccine against other strains. The results may not be generalizable to settings where an extended dosing interval was applied.
Health behaviors like social distancing and mask-wearing may confound the results obtained in this study.
- Mizrahi, B., Lotan, R., Kalkstein, N., et al. (2021). Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nature Communications 12(1), 6379. doi:10.1038/s41467-021-26672-3
Posted in: Medical Research News | Disease/Infection News | Healthcare News | Pharmaceutical News
Tags: Antibody, Assay, Coronavirus, Coronavirus Disease COVID-19, Efficacy, Healthcare, Polymerase, Polymerase Chain Reaction, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine

Written by
Dr. Shital Sarah Ahaley
Dr. Shital Sarah Ahaley is a medical writer. She completed her Bachelor's and Master's degree in Microbiology at the University of Pune. She then completed her Ph.D. at the Indian Institute of Science, Bengaluru where she studied muscle development and muscle diseases. After her Ph.D., she worked at the Indian Institute of Science, Education, and Research, Pune as a post-doctoral fellow. She then acquired and executed an independent grant from the DBT-Wellcome Trust India Alliance as an Early Career Fellow. Her work focused on RNA binding proteins and Hedgehog signaling.
Source: Read Full Article